

Chinese Bulletin of Botany ›› 2015, Vol. 50 ›› Issue (6): 721-732.DOI: 10.11983/CBB14194 cstr: 32102.14.CBB14194
Previous Articles Next Articles
Qian Wu1, Shuai Shao1, Shanshan Li2,3, Huijin Zhang2, Liangsheng Wang2*
Received:2014-11-15
Accepted:2015-04-26
Online:2015-11-01
Published:2015-09-06
Contact:
Wang Liangsheng
About author:? These authors contributed equally to this paper
Qian Wu, Shuai Shao, Shanshan Li, Huijin Zhang, Liangsheng Wang. Composition of Flavonoids in Lotus Pollen[J]. Chinese Bulletin of Botany, 2015, 50(6): 721-732.
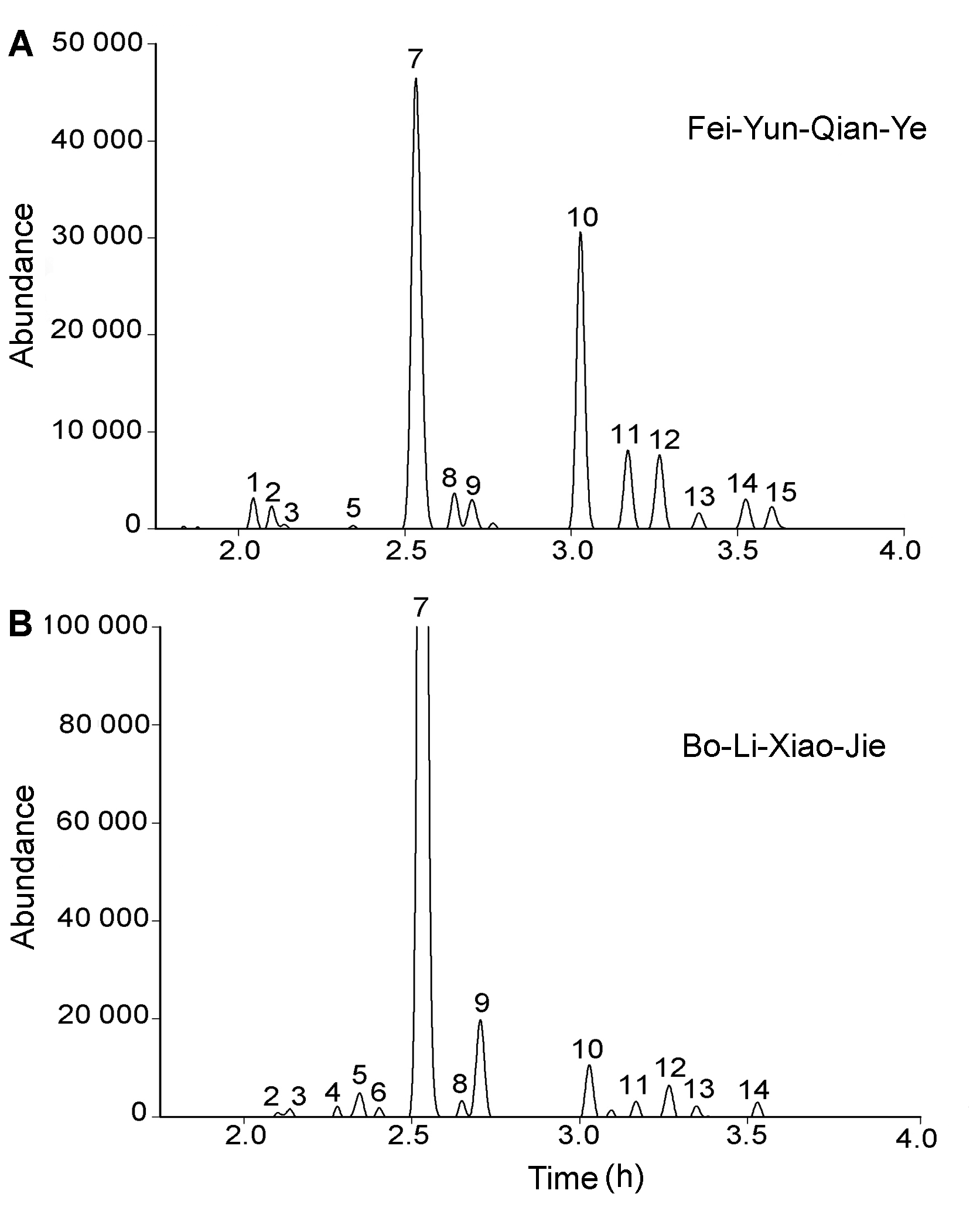
Figure 1 The UPLC profiles of flavonoids glycosides in pollen of Fei-Yun-Qian-Ye (A) and Bo-Li-Xiao-Jie (B) The detection wavelength is 350 nm, and the Bo-Li-Xiao-Jie’s is enlarged 4 times.
| Peak No. | Rt (min) | UV λmax (nm) | ESI-NI (m/z) | ESI-PI (m/z) | Identification | References |
|---|---|---|---|---|---|---|
| 1 | 2.04 | 248, 354 | 493[M-H]-, 317[A-H]- | 495[M+H]+, 319[A+H]+ | Myricetin 3-O-glucuronide | Chen et al., 2012a |
| 2 | 2.18 | 248, 352 | 479[M-H]--, 316[A-2H]- | 481[M+H]+, 319[A+H]+ | Myricetin 3-O-glucoside | Deng et al., 2013 |
| 3 | 2.23 | 248, 348 | 595[M-H]-, 300[A-2H]- | 597[M+H]+, 303[A+H]+ | Quercetin 3-O-arabino- pyranosyl-(1→2)-galacto- pyranoside | Chen et al., 2012a |
| 4 | 2.37 | 248, 348 | 298[A-2H]- | 301[A+H]+ | Diosmetin derivative | |
| 5 | 2.44 | 248, 348 | 477[M-H]-, 314[A-2H]- | 479[M+H]+, 317[A+H]+ | Isorhamnetin 3-O-glucoside | Li et al., 2014 |
| 6 | 2.50 | 248, 348 | 607[M-H-CH3]-, 299[A-H]- | 463[M+H-146]+, 301[A+H]+ | Diosmetin 7-O-rhamnopy- ranosyl-(1→6)-glucopy- ranoside | Li et al., 2014 |
| 7 | 2.63 | 248, 354 | 477[M-H]-, 301[A-H]- | 479[M+H]+, 303[A+H]+ | Quercetin 3-O-glucuronide | Deng et al., 2009 |
| 8 | 2.74 | 248, 354 | 463[M-H]-, 300[A-2H]- | 487[M+Na]+, 465[M+H]+, 303[A+H]+ | Quercetin 3-O-galactoside | Deng et al., 2009 |
| 9 | 2.80 | 248, 354 | 609[M-H]-, 301[A-H]- | 611[M+H]+, 303[A+H]+ | Quercetin 3-O-rhamnopy- ranosyl-(1→2)-glucopy- ranoside | Li et al., 2014 |
| 10 | 3.13 | 248, 347 | 447[M-H]-, 284[A-2H]- | 449[M+H]+, 287[A+H]+ | Kaempferol 3-O-galactoside | Jung et al., 2003 |
| 11 | 3.27 | 248, 348 | 461[M-H]-, 285[A-H]- | 463[M+H]+, 287[A+H]+ | Kaempferol 3-O-glucuronide | Chen et al., 2012a |
| 12 | 3.37 | 248, 348 | 447[M-H]-, 284[A-2H]- | 471[M+Na]+, 287[A+H]+ | Kaempferol 3-O-glucoside | Yang et al., 2009 |
| 13 | 3.49 | 248, 353 | 623[M-H]-, 477[M-H-146]-, 315[A-H]- | 625[M+H]+, 479[M+H-146]+, 317[A+H]+ | Isorhamnetin 3-O-rhamnopy- ranosyl-(1→6)-glucopy- ranoside | Chen et al., 2012a |
| 14 | 3.63 | 248, 354 | 477[M-H]-, 314[A-2H]- | 479[M+H]+, 317[A+H]+ | Isorhamnetin 3-O-hexose | Lim et al., 2006 |
| 15 | 3.71 | 248, 358 | 507[M-H]-, 344[A-2H]- | 509[M+H]+, 347[A+H]+ | Syringetin 3-O-glucoside | Guo et al., 2009 |
Table 1 I-Class UPLC/Xevo TQ MS analysis as well as the structure characterization and tentative identification of flavonols and flavonoids in lotus pollen
| Peak No. | Rt (min) | UV λmax (nm) | ESI-NI (m/z) | ESI-PI (m/z) | Identification | References |
|---|---|---|---|---|---|---|
| 1 | 2.04 | 248, 354 | 493[M-H]-, 317[A-H]- | 495[M+H]+, 319[A+H]+ | Myricetin 3-O-glucuronide | Chen et al., 2012a |
| 2 | 2.18 | 248, 352 | 479[M-H]--, 316[A-2H]- | 481[M+H]+, 319[A+H]+ | Myricetin 3-O-glucoside | Deng et al., 2013 |
| 3 | 2.23 | 248, 348 | 595[M-H]-, 300[A-2H]- | 597[M+H]+, 303[A+H]+ | Quercetin 3-O-arabino- pyranosyl-(1→2)-galacto- pyranoside | Chen et al., 2012a |
| 4 | 2.37 | 248, 348 | 298[A-2H]- | 301[A+H]+ | Diosmetin derivative | |
| 5 | 2.44 | 248, 348 | 477[M-H]-, 314[A-2H]- | 479[M+H]+, 317[A+H]+ | Isorhamnetin 3-O-glucoside | Li et al., 2014 |
| 6 | 2.50 | 248, 348 | 607[M-H-CH3]-, 299[A-H]- | 463[M+H-146]+, 301[A+H]+ | Diosmetin 7-O-rhamnopy- ranosyl-(1→6)-glucopy- ranoside | Li et al., 2014 |
| 7 | 2.63 | 248, 354 | 477[M-H]-, 301[A-H]- | 479[M+H]+, 303[A+H]+ | Quercetin 3-O-glucuronide | Deng et al., 2009 |
| 8 | 2.74 | 248, 354 | 463[M-H]-, 300[A-2H]- | 487[M+Na]+, 465[M+H]+, 303[A+H]+ | Quercetin 3-O-galactoside | Deng et al., 2009 |
| 9 | 2.80 | 248, 354 | 609[M-H]-, 301[A-H]- | 611[M+H]+, 303[A+H]+ | Quercetin 3-O-rhamnopy- ranosyl-(1→2)-glucopy- ranoside | Li et al., 2014 |
| 10 | 3.13 | 248, 347 | 447[M-H]-, 284[A-2H]- | 449[M+H]+, 287[A+H]+ | Kaempferol 3-O-galactoside | Jung et al., 2003 |
| 11 | 3.27 | 248, 348 | 461[M-H]-, 285[A-H]- | 463[M+H]+, 287[A+H]+ | Kaempferol 3-O-glucuronide | Chen et al., 2012a |
| 12 | 3.37 | 248, 348 | 447[M-H]-, 284[A-2H]- | 471[M+Na]+, 287[A+H]+ | Kaempferol 3-O-glucoside | Yang et al., 2009 |
| 13 | 3.49 | 248, 353 | 623[M-H]-, 477[M-H-146]-, 315[A-H]- | 625[M+H]+, 479[M+H-146]+, 317[A+H]+ | Isorhamnetin 3-O-rhamnopy- ranosyl-(1→6)-glucopy- ranoside | Chen et al., 2012a |
| 14 | 3.63 | 248, 354 | 477[M-H]-, 314[A-2H]- | 479[M+H]+, 317[A+H]+ | Isorhamnetin 3-O-hexose | Lim et al., 2006 |
| 15 | 3.71 | 248, 358 | 507[M-H]-, 344[A-2H]- | 509[M+H]+, 347[A+H]+ | Syringetin 3-O-glucoside | Guo et al., 2009 |
| [1] | 陈莲君 (2012). 油菜蜂花粉活性成分的提取分离及结构鉴定. 硕士论文. 无锡: 江南大学. pp. 16-32. |
| [2] | 董捷, 张红城, 李洁, 李慧 (2008). 八种蜂花粉醇提物中总多酚和总黄酮含量测定. 食品工业科技 29, 80-83. |
| [3] | 樊柏林, 王护民, 宋毅, 田辉, 李新兰 (2006). 破壁松花粉对高脂血症人群降血脂作用的观察. 职业与健康 22, 2012-2014. |
| [4] | 郭丽梅, 王盼盼 (2013). 板栗花粉黄酮的精制及其质谱分析. 天津科技大学学报 28(2), 15-18. |
| [5] | 蒋枫, 朱威, 张颖, 胡福良 (2007). 莲花花粉营养成分分析. 中国蜂业 58(9), 5-10. |
| [6] | 金高娃, 章飞芳, 薛兴亚, 肖远胜, 徐青, 梁鑫淼 (2006). 超高效液相色谱在复杂体系中药分离分析中的应用. 世界科学技术—中医药现代化 8, 106-111. |
| [7] | 李珊珊, 吴倩, 袁茹玉, 邵帅, 张会金, 王亮生 (2014). 莲属植物类黄酮代谢产物的研究进展. 植物学报 49, 738-750. |
| [8] | 林宣贤 (2007). 荷花黄酮类的提取及其生物活性的研究. 中国食品添加剂 (3), 65-68. |
| [9] | 饶剑, 吴盼盼, 徐德平 (2014). 油菜蜂花粉的抗氧化活性. 食品与发酵工业 40, 139-142. |
| [10] | 阮征, 邓泽元, 吴龙耀, 谢明勇, 吴谋成 (2008). HPLC法测定油菜蜂花粉中黄酮含量及六种破壁方法对黄酮提取的影响. 食品科学 29, 455-458. |
| [11] | 王开发 (2004). 花粉的功能与应用. 北京: 化学工业出版社. pp. 119-130. |
| [12] | 张行言 (陈龙清译) (2011). 中国荷花新品种图志I. 北京: 中国林业出版社. pp. 20-28. |
| [13] | 赵容 (2014). UPLC在药物分析中的应用. 黑龙江医药 27, 283-285. |
| [14] | 周顺华, 陶乐仁, 徐斐, 刘宝林, 郭旭峰 (2002). 用液氮淬冷法进行花粉破壁的实验研究. 上海理工大学学报 24, 233-237. |
| [15] | Ablajan K, Abliz Z, Shang XY, He JM, Zhang RP, Shi JG (2006). Structural characterization of flavonol 3,7-di-O- glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry.J Mass Spectrom 41, 352-360. |
| [16] | Agnihotri VK, ElSohly HN, Khan SI, Jacob MR, Joshi VC, Smillie T, Khan IA, Walker LA (2008). Constituents of Nelumbo nucifera leaves and their antimalarial and anti- fungal activity.Phytochem Lett 1, 89-93. |
| [17] | Chen S, Fang LC, Xi HF, Guan L, Fang JB, Liu YL, Wu BH, Li SH (2012a). Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectro- metry.Anal Chim Acta 724, 127-135. |
| [18] | Chen S, Wu BH, Fang JB, Liu YL, Zhang HH, Fang LC, Guan L, Li SH (2012b). Analysis of flavonoids from lotus (Nelumbo nucifera) leaves using high performance liquid chromatography/photodiode array detector tandem elec- trospray ionization mass spectrometry and an extraction method optimized by orthogonal design.J Chromatogr A 1227, 145-153. |
| [19] | Deng J, Chen S, Yin XJ, Wang K, Liu YL, Li SH, Yang PF (2013). Systematic qualitative and quantitative assess- ment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars.Food Chem 139, 307-312. |
| [20] | Deng SG, Deng ZY, Fan YW, Peng Y, Li J, Xiong DM, Liu R (2009). Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (lotus) by high-speed counter-current chromatography.J Chroma- togr B 877, 2487-2492. |
| [21] | Ferreres F, Pereira DM, Valentão P, Andrade PB (2010). First report of non-coloured flavonoids in Echium plant- agineum bee pollen: differentiation of isomers by liquid chromatography/ion trap mass spectrometry.Rapid Commun Mass Spectrom 24, 801-806. |
| [22] | Goo HR, Choi JS, Na DH (2009). Simultaneous determina- tion of quercetin and its glycosides from the leaves of Nelumbo nucifera by reversed-phase high-performance liquid chromatography.Arch Pharm Res 32, 201-206. |
| [23] | Goupy P, Vian MA, Chemat F, Caris-Veyrat C (2013). Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections.Ind Crop Prod 44, 496-510. |
| [24] | Guo XF, Wang DJ, Duan WJ, Du JH, Wang X (2009). Preparative isolation and purification of four flavonoids from the petals of Nelumbo nucifera by high-speed counter-current chromatography.Phytochem Anal 21, 268-272. |
| [25] | Huang B, Ban XQ, He JS, Tong J, Tian J, Wang YW (2010). Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves.Food Chem 120, 873-878. |
| [26] | Jung HA, Kim JE, Chung HY, Choi JS (2003). Antioxidant principles of Nelumbo nucifera stamens.Arch Pharm Res 26, 279-285. |
| [27] | Kashiwada Y, Aoshima A, Ikeshiro Y, Chen YP, Fur- ukawa H, Itoigawa M, Fujioka T, Mihashi K, Cosentino LM, Morris-Natschke SL, Lee KH (2005). Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity co- rrelations with related alkaloids.Bioorg Med Chem 13, 443-448. |
| [28] | Kim MJ, Shin HS (2012). Antioxidative effect of lotus seed and seedpod extracts.Food Sci Biotechnol 21, 1761-1766. |
| [29] | Klimczak I, Gliszczyńska-Świgło A (2015). Comparison of UPLC and HPLC methods for determination of vitamin C.Food Chem 175, 100-105. |
| [30] | Kredy HM, Huang DH, Xie BJ, He H, Yang E, Tian BQ, Xiao D (2010). Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential.Eur Food Res Technol 231, 387-394. |
| [31] | Li SS, Wu J, Chen LG, Du H, Xu YJ, Wang LJ, Zhang HJ, Zheng XC, Wang LS (2014). Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in lotus (Nelumbo nucifera).PLoS One 9, e108860. |
| [32] | Lim SS, Jung YJ, Hyun SK, Lee YS, Choi JS (2006). Rat lens aldose reductase inhibitory constituents of Nelumbo nucifera stamens.Phytother Res 20, 825-830. |
| [33] | Markham KR, Campos M (1996). 7- and 8-O-methylh- erbacetin-3-O-sophorosides from bee pollens and some structure/activity observations.Phytochemistry 43, 763-767. |
| [34] | Moon JH, Tsushida T, Nakahara K, Terao J (2001). Iden- tification of quercetin 3-O-β-D-glucuronide as an antio- xidative metabolite in rat plasma after oral administration of quercetin.Free Radic Biol Med 30, 1274-1285. |
| [35] | Ohkoshi E, Miyazaki H, Shindo K, Watanabe H, Yoshida A, Yajima H (2007). Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice.Planta Med 73, 1255-1259. |
| [36] | Pascoal A, Rodrigues S, Teixeira A, Feás X, Estevinho LM (2014). Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant and antiinflam- matory.Food Chem Toxicol 63, 233-239. |
| [37] | Qi SJ, Zhou DL (2013). Lotus seed epicarp extract as potential antioxidant and anti-obesity additive in Chinese Cantonese Sausage.Meat Sci 93, 257-262. |
| [38] | Su SL, Cui WX, Zhou W, Duan JA, Shang EX, Tang YP (2013). Chemical fingerprinting and quantitative constitu- ent analysis of Siwu decoction categorized formulae by UPLC-QTOF/MS/MS and HPLC-DAD.Chin Med 8, 5. |
| [39] | Tao WW, Yang NY, Duan JA, Wu DK, Guo JM, Tang YP, Qian DW, Zhu ZH (2011). Simultaneous determination of eleven major flavonoids in the pollen of Typha angustifolia by HPLC-PDA-MS.Phytochem Anal 22, 455-461. |
| [40] | Ulusoy E, Kolayli S (2014). Phenolic composition and antioxidant properties of anzer bee pollen.J Food Bio- chem 38, 73-82. |
| [41] | VanderMolen KM, Cech NB, Paine MF, Oberlies NH (2013). Rapid quantitation of furanocoumarins and flav- onoids in grapefruit juice using ultra-performance liquid chromatography.Phytochem Anal 24, 654-660. |
| [42] | Want EJ, Wilson ID, Gika H, Theodoridis G, Plumb RS, Shockcor J, Holmes E, Nicholson JK (2010). Global metabolic profiling procedures for urine using UPLC-MS.Nat Protoc 5, 1005-1018. |
| [43] | Wu XL, Prior RL (2005). Identification and characterization of anthocyanins by high-performance liquid chromato- graphy-electrospray ionization-tandem mass spectro- metry in common foods in the United States: vegetables, nuts, and grains.J Agric Food Chem 53, 3101-3113. |
| [44] | Yamazaki S, Miyoshi N, Kawabata K, Yasuda M, Shimoi K (2014). Quercetin-3-O-glucuronide inhibits noradrena- line-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β(2)-adrenergic signaling.Arch Biochem Biophys 557, 18-27. |
| [45] | Yang RZ, Wei XL, Gao FF, Wang LS, Zhang HJ, Xu YJ, Li CH, Ge YX, Zhang JJ, Zhang J (2009). Simultaneous analysis of anthocyanins and flavonols in petals of lotus (Nelumbo) cultivars by high-performance liquid chroma- tography-photodiode array detection/electrospray ioni- zation mass spectrometry.J Chromatogr A 1216, 106-112. |
| [46] | Zhang L, Shan Y, Tang KJ, Putheti R (2009). Ultrasound- assisted extraction flavonoids from lotus (Nelumbo nuficera Gaertn.) leaf and evaluation of its anti-fatigue activity.Inter J Physical Sci 4, 418-422. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||