

甘蓝型油菜BnaA02.CPSF6基因的克隆及功能分析(长英文摘要)
收稿日期: 2024-05-05
录用日期: 2024-07-23
网络出版日期: 2024-07-29
基金资助
国家自然科学基金(32071929);浙江省自然科学基金(LY21C130001);浙江省“十四五”农业新品种选育重大科技专项子课题(2021C02064-2-1);浙江农林大学科研发展基金(2021FR044)
Cloning and Functional Analysis of the BnaA02.CPSF6 Gene from Brassica napus
Received date: 2024-05-05
Accepted date: 2024-07-23
Online published: 2024-07-29
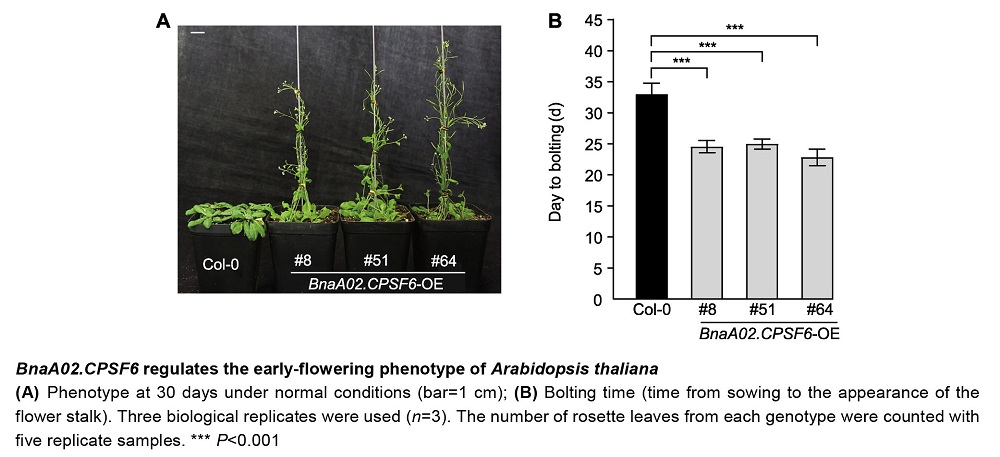
CPSF家族蛋白是植物体内mRNA前体中多聚腺苷酸化信号识别、剪切和添加poly(A)的重要因子, 对开花时间调控、环境响应和种子发育等具有重要作用。目前, 甘蓝型油菜(Brassica napus) CPSF家族基因的功能尚不明确。为探究甘蓝型油菜CPSF家族基因的功能和表达模式, 从甘蓝型油菜品种中双11号中克隆得到BnaA02.CPSF6, 并对其进行生物信息学、编码蛋白质亚细胞定位、表达模式和基因功能分析。结果表明, BnaA02.CPSF6基因编码区全长1 938 bp, 编码646个氨基酸残基, 无内含子结构, 其在甘蓝型油菜中有6个同源基因; BnaA02.CPSF6启动子区存在多个参与光反应的顺式作用元件和MYB结合位点; BnaA02.CPSF6在根、茎、叶、花和不同发育时期种子中均有表达, 特别是在发育15-35天的种子中显著高表达, 其编码的蛋白定位于细胞核; BnaA02.CPSF6受盐和干旱胁迫诱导上调表达; 在ABA、IAA、GA3、SA和MeJA激素处理下, BnaA02.CPSF6基因表达先受到抑制再逐渐恢复至正常水平; 在正常条件下, 在拟南芥(Arabidopsis thaliana)中过表达BnaA02.CPSF6会出现提前抽薹开花的表型, 且莲座叶数量显著减少。综上所述, BnaA02.CPSF6参与非生物胁迫响应并受植物激素调控, 可能在开花调控中起促进作用。

关键词: 甘蓝型油菜; BnaA02.CPSF6; 基因功能; 表达模式; 生长发育
李青洋 , 刘翠 , 何李 , 彭姗 , 马嘉吟 , 胡子祎 , 刘宏波 . 甘蓝型油菜BnaA02.CPSF6基因的克隆及功能分析(长英文摘要)[J]. 植物学报, 2025 , 60(1) : 62 -73 . DOI: 10.11983/CBB24068
INTRODUCTION: The CPSF family (cleavage and polyadenylation specificity factor) is a crucial protein family that is responsible for polyadenylation signal recognition in mRNA precursors, cleavage and the addition of poly(A) tails to mRNAs in plants. This family plays crucial roles in the regulation of flowering time, the environmental response, and seed development. Currently, the function of the CPSF family genes in Brassica napus is unclear.
RATIONALE: To explore the function and expression patterns of the CPSF gene family, this study cloned BnaA02.CPSF6 from B. napus variety Zhongshuang No.11 and conducted bioinformatics analysis, subcellular localization, expression pattern, and functional characterization of the gene.
RESULTS: These results indicate that the coding region of the BnaA02.CPSF6 gene is 1 938 bp in length and encodes 646 amino acids without intron structures. Its promoter region contains multiple cis-acting elements involved in light responses and MYB binding sites. Additionally, there are six genes homologous to BnaA02.CPSF6 in B. napus. The BnaA02.CPSF6 gene expressed in the roots, stems, leaves, flowers and different developmental seeds of B. napus, especially significantly higher in 15-35 d developmental seeds, and its encoded protein was localized in the nucleus. The BnaA02.CPSF6 gene expression is upregulated under salt and drought stress. Under treatment with hormones such as ABA, IAA, GA3, SA, and MeJA, the expression of BnaA02.CPSF6 gene is initially inhibited and then gradually recovers to normal levels. Under normal conditions, the overexpression of the BnaA02.CPSF6 gene in Arabidopsis thaliana results in an early bolting phenotype, along with a reduced number of rosette leaves.
CONCLUSION: In summary, the above results indicate that the BnaA02.CPSF6 is involved in abiotic stress responses, is regulated by phytohormones, and may also play a promoting role in flowering regulation.

| [1] | Bao SJ, Hua CM, Shen LS, Yu H (2020). New insights into gibberellin signaling in regulating flowering in Arabidopsis. J Integr Plant Biol 62,118-131. |
| [2] | Boreikait? V, Passmore LA (2023). 3′-end processing of eukaryotic mRNA: machinery, regulation, and impact on gene expression. Annu Rev Biochem 92, 199-225. |
| [3] | Brown KM, Gilmartin GM (2003). A mechanism for the regulation of pre-mRNA 3′ processing by human cleavage factor Im. Mol Cell 12, 1467-1476. |
| [4] | Cai FF, Shao CS, Sun YQ (2022). The role of alternative splicing in floral transition. Chin Bull Bot 57, 69-79. (in Chinese) |
| 蔡芳芳, 邵长生, 孙玉强 (2022). 可变剪切在植物成花转换中的作用. 植物学报 57, 69-79. | |
| [5] | Cui GX, Hou J, Tong L, Xu ZR (2010). Light responsive elements and binding proteins of plant genes. Plant Physiol Commun 46, 991-1000. (in Chinese) |
| 崔国新, 侯杰, 佟玲, 许志茹 (2010). 植物基因光反应元件及其结合蛋白. 植物生理学通讯 46, 991-1000. | |
| [6] | Dai YQ, Luo LJ, Zhao Z (2023). Genetic robustness control of auxin output in priming organ initiation. Proc Natl Acad Sci USA 120, e2221606120. |
| [7] | Eckardt NA (2002). Alternative splicing and the control of flowering time. Plant Cell 14, 743-747. |
| [8] | Edwalds-Gilbert G, Milcarek C (1995). The binding of a subunit of the general polyadenylation factor cleavage- polyadenylation specificity factor (CPSF) to polyadenylation sites changes during B cell development. Nucleic Acids Symp Ser (33), 229-233. |
| [9] | Feng W, Jacob Y, Veley KM, Ding L, Yu XH, Choe G, Michaels SD (2011). Hypomorphic alleles reveal FCA- independent roles for FY in the regulation of FLOWERING LOCUS C. Plant Physiol 155, 1425-1434. |
| [10] | Hao SQ, Zhang LD, Zhao DH, Zhou JW, Ye CT, Qu HD, Li QQ (2023). Inhibitor AN3661 reveals biological functions of Arabidopsis CLEAVAGE and POLYADENYLATION SPECIFICITY FACTOR 73. Plant Physiol 193, 537-554. |
| [11] | Hardy JG, Norbury CJ (2016). Cleavage factor Im (CFIm) as a regulator of alternative polyadenylation. Biochem Soc Trans 44, 1051-1057. |
| [12] | Henderson IR, Liu FQ, Drea S, Simpson GG, Dean C (2005). An allelic series reveals essential roles for FY in plant development in addition to flowering-time control. Development 132, 3597-3607. |
| [13] | Herr AJ, Molnàr A, Jones A, Baulcombe DC (2006). Defective RNA processing enhances RNA silencing and influences flowering of Arabidopsis. Proc Natl Acad Sci USA 103, 14994-15001. |
| [14] | Hornyik C, Terzi LC, Simpson GG (2010). The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell 18(2), 203-213. |
| [15] | Hou YF, Sun J, Wu BX, Gao YY, Nie HB, Nie ZT, Quan SX, Wang Y, Cao XF, Li SS (2021). CPSF30-L-mediated recognition of mRNA m6A modification controls alternative polyadenylation of nitrate signaling-related gene transcripts in Arabidopsis. Mol Plant 14, 688-699. |
| [16] | Huang Y, Zhao PS, Xie LL, Xu JS, Cheng Y, Zhang XK, Xu BB (2024). Analysis on yield composition and breeding strategy of winter rape varieties in the Yangtze River Basin. Chin J Oil Crop Sci 46, 13-18. (in Chinese) |
| 黄郢, 赵培森, 谢伶俐, 徐劲松, 程勇, 张学昆, 许本波 (2024). 长江流域冬油菜品种产量构成及育种策略分析. 中国油料作物学报 46, 13-18. | |
| [17] | Kumar A, Clerici M, Muckenfuss LM, Passmore LA, Jinek M (2019). Mechanistic insights into mRNA 3′-end processing. Curr Opin Struct Biol 59, 143-150. |
| [18] | Li GC, Niu QC, Leng BF, Ding YF, Tong T, Fan LX (2024). The decade of rapeseed industry in the new era: development and its path choice. Chin J Oil Crop Sci 46, 228-235. (in Chinese) |
| 李谷成, 牛秋纯, 冷博峰, 丁逸飞, 童婷, 范丽霞 (2024). 新时代十年: 我国油菜产业发展与路径选择. 中国油料作物学报 46, 228-235. | |
| [19] | Li QX, Zhang L, Wang Y, Huang XX (2019). The research progress of gibberellin on the regulation of flowering and floral organ development in plant. Chin J Cell Biol 41, 746-758. (in Chinese) |
| 李巧峡, 张丽, 王玉, 黄小霞 (2019). 赤霉素调控植物开花及花器官发育的研究进展. 中国细胞生物学学报 41, 746-758. | |
| [20] | Lin JC, Xu RQ, Wu XH, Shen YJ, Li QQ (2017). Role of cleavage and polyadenylation specificity factor 100: anchoring poly (A) sites and modulating transcription termination. Plant J 91, 829-839. |
| [21] | Liu YT, Wu GX, Zhao YP, Wang HHL, Dai ZY, Xue WC, Yang J, Wei HB, Shen RX, Wang HY (2021). DWARF53 interacts with transcription factors UB2/UB3/TSH4 to regulate maize tillering and tassel branching. Plant Physiol 187, 947-962. |
| [22] | Ma LY, Guo C, Li QQ (2014). Role of alternative polyadenylation in epigenetic silencing and antisilencing. Proc Natl Acad Sci USA 111, 9-10. |
| [23] | Macknight R, Duroux M, Laurie R, Dijkwel P, Simpson G, Dean C (2002). Functional significance of the alternative transcript processing of the Arabidopsis floral promoter FCA. Plant Cell 14, 877-888. |
| [24] | Mandel CR, Bai Y, Tong L (2008). Protein factors in pre- mRNA 3′-end processing. Cell Mol Life Sci 65, 1099-1122. |
| [25] | Mouradov A, Cremer F, Coupland G (2002). Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 14, S111-S130. |
| [26] | Proudfoot N (2004). New perspectives on connecting messenger RNA 3′ end formation to transcription. Curr Opin Cell Biol 16, 272-278. |
| [27] | Quesada V, Macknight R, Dean C, Simpson GG (2003). Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time. EMBO J 22, 3142-3152. |
| [28] | Schul W, Groenhout B, Koberna K, Takagaki Y, Jenny A, Manders EM, Raska I, van Driel R, de Jong L (1996). The RNA 3′ cleavage factors CstF 64 kDa and CPSF 100 kDa are concentrated in nuclear domains closely associated with coiled bodies and newly synthesized RNA. EMBO J 15, 2883-2892. |
| [29] | Song PZ, Yang JB, Wang CL, Lu Q, Shi LQ, Tayier S, Jia GF (2021). Arabidopsis N6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol Plant 14, 571-587. |
| [30] | Thomas PE, Wu XH, Liu M, Gaffney B, Ji GL, Li QQ, Hunt AG (2012). Genome-wide control of polyadenylation site choice by CPSF30 in Arabidopsis. Plant Cell 24, 4376-4388. |
| [31] | Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, Meinke D (2004). Identification of genes required for embryo development in Arabidopsis. Plant J 135, 1206-1220. |
| [32] | Venkataraman K, Brown KM, Gilmartin GM (2005). Analysis of a noncanonical poly (A) site reveals a tripartite mechanism for vertebrate poly (A) site recognition. Genes Dev 19, 1315-1327. |
| [33] | Wang XP, Niu YL, Zheng Y (2021). Multiple functions of MYB transcription factors in abiotic stress responses. Int J Mol Sci 22, 6125. |
| [34] | Yang Q, Coseno M, Gilmartin GM, Doublié S (2011). Crystal structure of a human cleavage factor CFIm25/CFIm68/RNA complex provides an insight into poly(A) site recognition and RNA looping. Structure 19, 368-377. |
| [35] | Yu ZB, Lin JC, Li QQ (2019). Transcriptome analyses of FY mutants reveal its role in mRNA alternative polyadenylation. Plant Cell 31, 2332-2352. |
| [36] | Zhang CS, Wei T, Zhou YP, Fan T, Lü TX, Tian CE (2021). Progress in flowering regulation mechanisms of FLC. Chin Bull Bot 56,651-663. (in Chinese) |
| 张长生, 魏滔, 周玉萍, 范甜, 吕天晓, 田长恩 (2021). FLC调控植物成花的分子机制研究新进展. 植物学报 56, 651-663. | |
| [37] | Zhang XJ, Nomoto M, Garcia-León M, Takahashi N, Kato M, Yura K, Umeda M, Rubio V, Tada Y, Furumoto T, Aoyama T, Tsuge T (2022). CFI 25 subunit of cleavage factor I is important for maintaining the diversity of 3′ UTR lengths in Arabidopsis thaliana (L.) heynh. Plant Cell Physiol 63, 369-383. |
/
| 〈 |
|
〉 |