

水稻突变体pe-1对弱光胁迫的响应机制
收稿日期: 2024-03-11
录用日期: 2024-05-07
网络出版日期: 2024-05-07
基金资助
浙江省自然科学基金重点项目(LZ23C130003);2024年国家级大学生创新创业训练计划和2024年浙江省大学生科技创新活动计划暨新苗人才计划
Response Mechanism of Rice Mutant pe-1 to Low Light Stress
Received date: 2024-03-11
Accepted date: 2024-05-07
Online published: 2024-05-07
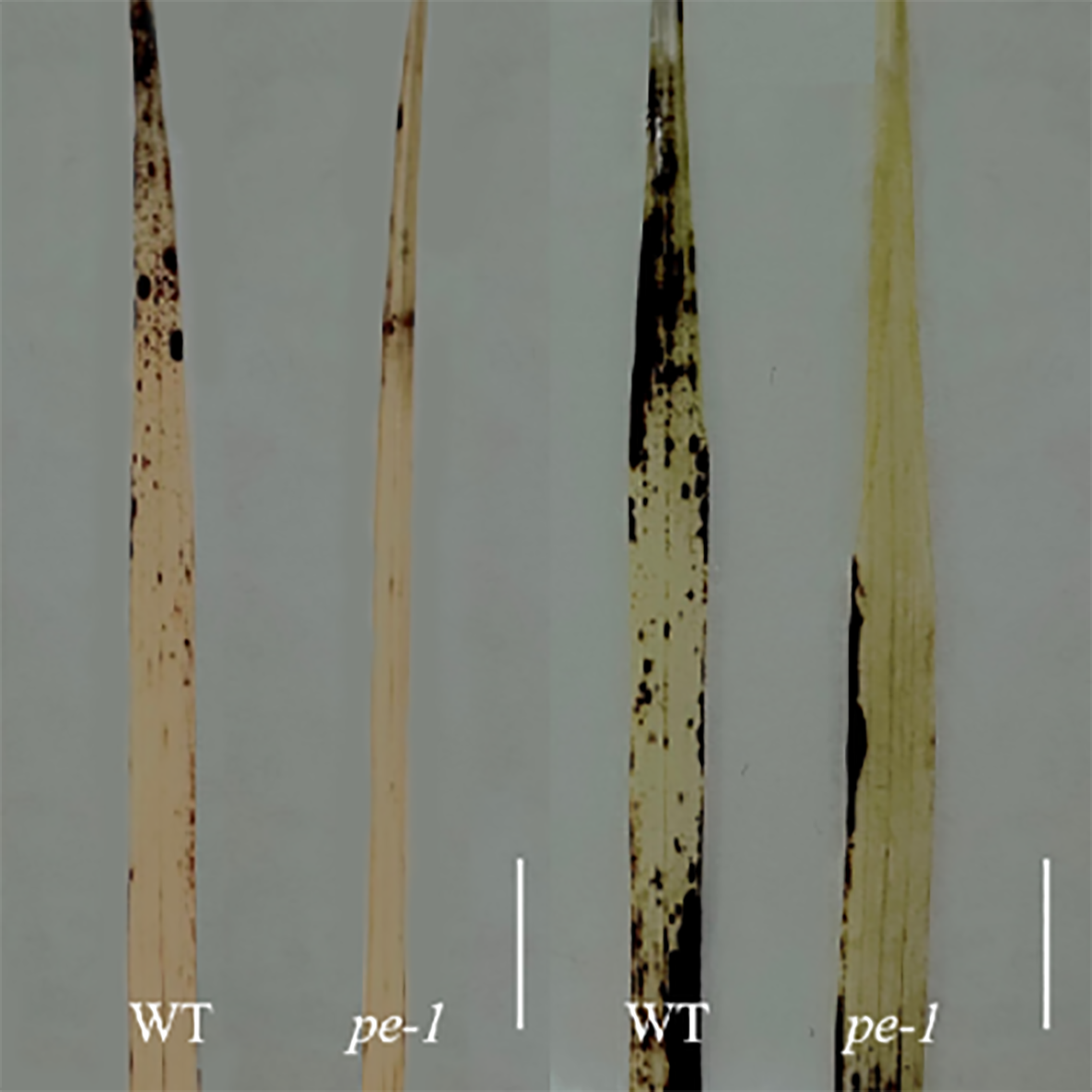
以γ射线诱变籼稻双科早(Oryza sativa subsp. indica cv. ‘Shuangkezao’)获得的早熟鲜绿突变体pe-1为实验材料, 在三叶期和分蘖期进行弱光胁迫, 探讨pe-1与野生型在形态特征、非生物胁迫相关酶活性及其调控基因表达量、叶绿素含量、叶绿体合成与降解及光形态建成相关基因表达对弱光响应的差异。结果表明, 与野生型相比, 弱光胁迫后, pe-1叶片黄化程度显著降低, 株高和叶面积显著增加; 三叶期和分蘖期的叶片中不同叶绿素含量变化不同。此外, pe-1叶绿素含量增加, 且其抗氧化应激反应相关酶过氧化氢酶(CAT)和过氧化物酶(POD)的活性及相关基因的表达量均高于野生型, 表明在弱光胁迫下pe-1活性氧清除能力增强, 适应能力更强。pe-1的光形态建成相关基因表达量高于野生型, 表明弱光处理下pe-1的光接收能力更强。综上, pe-1突变体具有抵御弱光胁迫的潜力, 该结果有助于耐弱光水稻品种的选育。
黄佳慧 , 杨惠敏 , 陈欣雨 , 朱超宇 , 江亚楠 , 胡程翔 , 连锦瑾 , 芦涛 , 路梅 , 张维林 , 饶玉春 . 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024 , 59(4) : 574 -584 . DOI: 10.11983/CBB24039
This study utilized the γ-ray-induced early-maturation, fresh-green mutant line pe-1 from indica rice as an experimental material. At the trilobal stage and the tillering stage, we observed differences in morphological characteristics between pe-1 and wild type. In addition, we measured the activity of antioxidant-related enzymes and their regulatory genes expression, chlorophyll content and chloroplast synthesis and degradation-related gene expression, and photomorphogenesis-related gene expression to detect the differences in the low light response between the pe-1 and wild type. The results showed that pe-1 exhibited less leaf yellowing, taller stature, and larger leaf area compared to wild type post-stress. The changes in chlorophyll content differed between leaves at the trilobal stage and the tillering stage. Additionally, pe-1 resulted in increased chlorophyll content and elevated levels of the stress-responsive enzymes catalase and peroxidase, as well as increased expression of related genes. This indicates enhanced reactive oxygen species sca- venging and stronger adaptability to adverse conditions under low light conditions. Moreover, pe-1 exhibited increased expression levels of genes associated with photomorphogenesis, indicating superior light perception ability under low light intensities. In summary, the pe-1 mutant shows immense potential for survival under low light stress, contributing to the breeding rice with low light tolerance.

| [1] | 陈德良, 陶月良, 吴友贵, 程瑶, 夏家天 (2016). 遮荫对百山祖冷杉光合特性和叶绿素荧光参数的影响. 核农学报 30, 2056-2064. |
| [2] | 陈小玲, 陈清西 (2014). 植物弱光逆境生理的研究进展. 北方园艺 (6), 183-187. |
| [3] | 陈宇彬 (2023). 中国南方水稻复种指数时空演变及驱动机制分析. 硕士论文. 太原: 山西财经大学. pp. 19-21. |
| [4] | 种培芳 (2003). 弱光胁迫对甜瓜(Cucumis melo L.)光合特性及生长发育的影响. 硕士论文. 兰州: 甘肃农业大学. pp. 20-22. |
| [5] | 方希林, 杨漫, 王鑫, 黄沆, 肖楠, 贺治洲, 王悦 (2017). 水稻叶色突变体ygr的遗传分析与基因定位. 核农学报 31, 2096-2102. |
| [6] | 李学孚, 倪智敏, 吴月燕, 李美芹, 刘蓉, 饶慧云 (2015). 盐胁迫对‘鄞红’葡萄光合特性及叶片细胞结构的影响. 生态学报 35, 4436-4444. |
| [7] | 刘利, 王丽, 邓飞, 黄云, 刘代银, 任万军, 杨文钰 (2012). 遮荫对不同杂交稻组合叶片渗透调节物质含量及保护酶活性的影响. 中国水稻科学 26, 569-575. |
| [8] | 彭琦, 杨柳青 (2022). 弱光胁迫对白蟾生理特性的影响. 绿色科技 24(15), 179-184, 218. |
| [9] | 王学春, 赵祥, 赵长坤, 杨国涛, 彭友林, 胡运高 (2021). 四川常用杂交水稻对弱光胁迫的响应差异及其评价体系构建. 云南大学学报(自然科学版) 43, 386-394. |
| [10] | 薛伟, 李向义, 朱军涛, 林丽莎, 王迎菊 (2011). 遮荫对疏叶骆驼刺叶形态和光合参数的影响. 植物生态学报 35, 82- 90. |
| [11] | 严如玉, 甘国渝, 赵希梅, 殷大聪, 李燕丽, 金慧芳, 朱海, 李继福 (2023). 我国水稻优势产区生产格局及施肥现状研究. 中国稻米 29(3), 1-8. |
| [12] | 张彩霞 (2016). 气候变化背景下南方主要种植制度的气候适宜性研究. 硕士论文. 南昌: 江西农业大学. pp. 13-18. |
| [13] | Bai B, Lu NN, Li YP, Guo SL, Yin HB, He YN, Sun W, Li W, Xie XZ (2019). OsBBX14 promotes photomorphogenesis in rice by activating OsHY5L1 expression under blue light conditions. Plant Sci 284, 192-202. |
| [14] | Das P, Lakra N, Nutan KK, Singla-Pareek SL, Pareek A (2019). A unique bZIP transcription factor imparting multiple stress tolerance in rice. Rice 12, 58. |
| [15] | Hirose F, Inagaki N, Hanada A, Yamaguchi S, Kamiya Y, Miyao A, Hirochika H, Takano M (2012). Cryptochrome and phytochrome cooperatively but independently reduce active gibberellin content in rice seedlings under light irradiation. Plant Cell Physiol 53, 1570-1582. |
| [16] | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-??CT method. Methods 25, 402-408. |
| [17] | Nikiforou C, Manetas Y (2011). Inherent nitrogen deficiency in Pistacia lentiscus preferentially affects photosystem I: a seasonal field study. Funct Plant Biol 38, 848-855. |
| [18] | Sato Y, Morita R, Katsuma S, Nishimura M, Tanaka A, Kusaba M (2009). Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J 57, 120-131. |
| [19] | Takano M, Inagaki N, Xie XZ, Kiyota S, Baba-Kasai A, Tanabata T, Shinomura T (2009). Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc Natl Acad Sci USA 106, 14705-14710. |
| [20] | Tsugane K, Maekawa M, Takagi K, Takahara H, Qian Q, Eun CH, Iida S (2006). An active DNA transposon nDart causing leaf variegation and mutable dwarfism and its related elements in rice. Plant J 45, 46-57. |
| [21] | Wang YL, Wang CM, Zheng M, Lyu J, Xu Y, Li XH, Niu M, Long WH, Wang D, Wang HY, Terzaghi W, Wang YH, Wan JM (2016). WHITE PANICLE1, a val-tRNA synthetase regulating chloroplast ribosome biogenesis in rice, is essential for early chloroplast development. Plant Physiol 170, 2110-2123. |
| [22] | Zhang HT, Li JJ, Yoo JH, Yoo SC, Cho SH, Koh HJ, Seo HS, Paek NC (2006). Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol 62, 325-337. |
| [23] | Zhang ZS, Lu YS, Zhai LG, Deng RS, Jiang J, Li Y, He ZH, Peng XX (2012). Glycolate oxidase isozymes are coordinately controlled by GLO1 and GLO4 in rice. PLoS One 7, e39658. |
| [24] | Zhang ZS, Xu YY, Xie ZW, Li XY, He ZH, Peng XX (2016). Association-dissociation of glycolate oxidase with catalase in rice: a potential switch to modulate intracellular H2O2 levels. Mol Plant 9, 737-748. |
| [25] | Zhao Q, Zhou LJ, Liu JC, Cao ZZ, Du XX, Huang FD, Pan G, Cheng FM (2018). Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep 37, 741-757. |
/
| 〈 |
|
〉 |