

植物非典型Aux/IAA蛋白应答生长素研究进展
收稿日期: 2023-08-03
录用日期: 2023-11-14
网络出版日期: 2023-12-04
基金资助
国家自然科学基金青年科学基金(31800213)
Research Progress on Auxin Responsive Non-canonical Aux/IAA Proteins in Plants
Received date: 2023-08-03
Accepted date: 2023-11-14
Online published: 2023-12-04
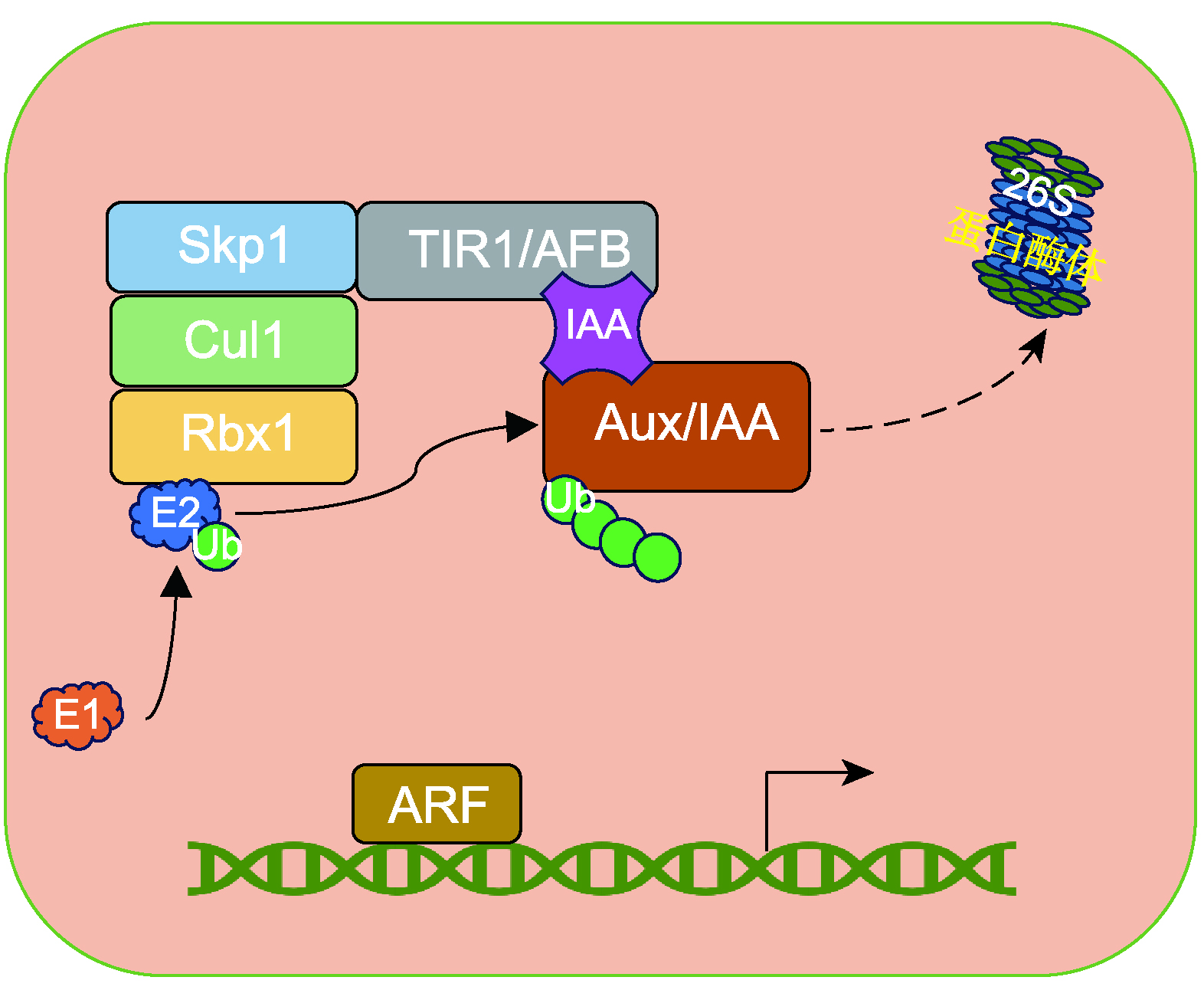
植物激素生长素调控植物生长发育及环境适应的多个过程, 包括胚胎发育、器官发生和向性生长等。生长素发挥生物学功能主要依赖于经典的TIR1/AFB-auxin-Aux/IAA-ARF信号转导途径。其中, 由4个保守结构域组成的典型Aux/IAA蛋白作为TIR1/AFB的共受体在生长素信号转导过程中发挥关键作用。然而, 近年来发现缺乏保守结构域的非典型Aux/IAA蛋白也参与生长素的应答与调控作用。该文从蛋白结构、生物学功能及参与生长素信号转导等方面综述了非典型Aux/IAA蛋白的研究进展, 探讨和展望了非典型Aux/IAA蛋白的研究方向。
关键词: 生长素; Aux/IAA蛋白; 非典型Aux/IAA蛋白; 结构与功能; 信号转导
周玉滢 , 陈辉 , 刘斯穆 . 植物非典型Aux/IAA蛋白应答生长素研究进展[J]. 植物学报, 2024 , 59(4) : 651 -658 . DOI: 10.11983/CBB23106
The plant hormone auxin regulates many processes of plant growth and development, including embryonic development, organogenesis, and tropism, as well as environmental adaptation. To perform these functions, auxin mainly depends on the typical TIR1/AFB-auxin-Aux/IAA-ARF signaling pathway. In this pathway, the canonical Aux/IAA proteins composed of four conserved domains play a key role in auxin signaling as co-receptor with TIR1/AFB. Recently, some non-canonical Aux/IAA proteins lacking conserved domain(s) were also found to be involved in the auxin response. This review focuses on the research advances of non-canonical Aux/IAA proteins on their structure, biological function and roles in auxin signal transduction, and discusses the future research directions of these proteins.

| [1] | Basunia MA, Nonhebel HM, Backhouse D, McMillan M (2021). Localised expression of OsIAA29 suggests a key role for auxin in regulating development of the dorsal aleurone of early rice grains. Planta 254, 40. |
| [2] | Béziat C, Kleine-Vehn J (2018). The road to auxin-dependent growth repression and promotion in apical hooks. Curr Biol 28, R519-R525. |
| [3] | Calderón Villalobos LIA, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao HB, Zheng N, Napier R, Kepinski S, Estelle M (2012). A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nat Chem Biol 8, 477-485. |
| [4] | Cao M, Chen R, Li P, Yu YQ, Zheng R, Ge DF, Zheng W, Wang XH, Gu YT, Gelová Z, Friml J, Zhang H, Liu RY, He J, Xu TD (2019). TMK1-mediated auxin signaling regulates differential growth of the apical hook. Nature 568, 240-243. |
| [5] | Chen H, Ma B, Zhou Y, He SJ, Tang SY, Lu X, Xie Q, Chen SY, Zhang JS (2018). E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proc Natl Acad Sci USA 115, 4513- 4518. |
| [6] | Dharmasiri N, Dharmasiri S, Estelle M (2005). The F-box protein TIR1 is an auxin receptor. Nature 435, 441-445. |
| [7] | Ding ZJ, Friml J (2010). Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci USA 107, 12046-12051. |
| [8] | Dreher KA, Brown J, Saw RE, Callis J (2006). The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18, 699-714. |
| [9] | Figueiredo MRA, Strader LC (2022). Intrinsic and extrinsic regulators of Aux/IAA protein degradation dynamics. Trends Biochem Sci 47, 865-874. |
| [10] | Friml J, Gallei M, Gelová Z, Johnson A, Mazur E, Monzer A, Rodriguez L, Roosjen M, Verstraeten I, ?ivanovi? BD, Zou MX, Fiedler L, Giannini C, Grones P, Hrtyan M, Kaufmann WA, Kuhn A, Narasimhan M, Randuch M, Rydza N, Takahashi K, Tan ST, Teplova A, Kinoshita T, Weijers D, Rakusová H (2022). ABP1-TMK auxin perception for global phosphorylation and auxin canalization. Nature 609, 575-581. |
| [11] | Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001). Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414, 271-276. |
| [12] | Guilfoyle TJ (2015). The PB1 domain in auxin response factor and Aux/IAA proteins: a versatile protein interaction module in the auxin response. Plant Cell 27, 33-43. |
| [13] | Han M, Park Y, Kim I, Kim EH, Yu TK, Rhee S, Suh JY (2014). Structural basis for the auxin-induced transcrip-tional regulation by Aux/IAA17. Proc Natl Acad Sci USA 111, 18613-18618. |
| [14] | Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP (2006). Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sati-va). Funct Integr Genomics 6, 47-59. |
| [15] | Jing HW, Yang XL, Emenecker RJ, Feng J, Zhang J, Chaisupa P, Wright RC, Holehouse AS, Strader LC, Zuo JR (2023). Nitric oxide-mediated S-nitrosylation of IAA17 protein in intrinsically disordered region represses auxin signaling. J Genet Genomics 50, 473-485. |
| [16] | Jing HW, Yang XL, Zhang J, Liu XH, Zheng HK, Dong GJ, Nian JQ, Feng J, Xia B, Qian Q, Li JY, Zuo JR (2015). Peptidyl-prolyl isomerization targets rice Aux/IAAs for proteasomal degradation during auxin signaling. Nat Com-mun 6, 7395. |
| [17] | Kepinski S, Leyser O (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435, 446-451. |
| [18] | Li LX, Gallei M, Friml J (2022). Bending to auxin: fast acid growth for tropisms. Trends Plant Sci 27, 440-449. |
| [19] | Li YY, Qi YH (2022). Advances in biological functions of Aux/IAA gene family in plants. Chin Bull Bot 57, 30-41. (in Chinese) |
| 李艳艳, 齐艳华 (2022). 植物Aux/IAA基因家族生物学功能研究进展. 植物学报 57, 30-41. | |
| [20] | Lin WW, Zhou X, Tang W, Takahashi K, Pan X, Dai JW, Ren H, Zhu XY, Pan SQ, Zheng HY, Gray WM, Xu TD, Kinoshita T, Yang ZB (2021). TMK-based cell-surface auxin signaling activates cell-wall acidification. Nature 599, 278-282. |
| [21] | Lv BS, Yu QQ, Liu JJ, Wen XJ, Yan ZW, Hu KQ, Li HB, Kong XP, Li CL, Tian HY, De Smet I, Zhang XS, Ding ZJ (2020). Non-canonical AUX/IAA protein IAA33 compe-tes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J 39, e101515. |
| [22] | Mockaitis K, Estelle M (2008). Auxin receptors and plant development: a new signaling paradigm. Annu Rev Cell Dev Biol 24, 55-80. |
| [23] | Müller CJ, Valdés AE, Wang GD, Ramachandran P, Beste L, Uddenberg D, Carlsbecker A (2016). PHABULOSA mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis. Plant Physiol 170, 956-970. |
| [24] | Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GKS, Weijers D (2018). Origin and evolution of the nuclear auxin response system. eLife 7, e33399. |
| [25] | Nanao MH, Vinos-Poyo T, Brunoud G, Thévenon E, Mazzoleni M, Mast D, Lainé S, Wang SC, Hagen G, Li HB, Guilfoyle TJ, Parcy F, Vernoux T, Dumas R (2014). Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun 5, 3617. |
| [26] | Park J, Kim YS, Kim SG, Jung JH, Woo JC, Park CM (2011). Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol 156, 537-549. |
| [27] | Piya S, Shrestha SK, Binder B, Stewart CN Jr, Hewezi T (2014). Protein-protein interaction and gene co-expression maps of ARFs and Aux/IAAs in Arabidopsis. Front Plant Sci 5, 744. |
| [28] | Reed JW (2001). Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci 6, 420-425. |
| [29] | Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O (1998). Changes in auxin response from mutations in an AUX/IAA gene. Science 279, 1371-1373. |
| [30] | Salehin M, Bagchi R, Estelle M (2015). SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell 27, 9-19. |
| [31] | Sato A, Yamamoto KT (2008). Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol Plant 133, 397- 405. |
| [32] | Shimizu-Mitao Y, Kakimoto T (2014). Auxin sensitivities of all Arabidopsis Aux/IAAs for degradation in the presence of every TIR1/AFB. Plant Cell Physiol 55, 1450-1459. |
| [33] | Song YL, Xu ZF (2013). Ectopic overexpression of an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) gene OsIAA4 in rice induces morphological changes and reduces res-ponsiveness to auxin. Int J Mol Sci 14, 13645-13656. |
| [34] | Szemenyei H, Hannon M, Long JA (2008). TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319, 1384-1386. |
| [35] | Tan X, Calderon-Villalobos LIA, Sharon M, Zheng CX, Robinson CV, Estelle M, Zheng N (2007). Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640-645. |
| [36] | Teale WD, Paponov IA, Palme K (2006). Auxin in action: signaling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7, 847-859. |
| [37] | Teng ZN, Yu HH, Wang GQ, Meng S, Liu BH, Yi YK, Chen YK, Zheng Q, Liu L, Yang JC, Duan MJ, Zhang JH, Ye NH (2022). Synergistic interaction between ABA and IAA due to moderate soil drying promotes grain filling of infe-rior spikelets in rice. Plant J 109, 1457-1472. |
| [38] | Tiwari SB, Hagen G, Guilfoyle TJ (2004). Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16, 533-543. |
| [39] | Vain T, Raggi S, Ferro N, Barange DK, Kieffer M, Ma Q, Doyle SM, Thelander M, Pa?ízková B, Novák O, Ismail A, Enquist PA, Rigal A, ?angowska M, Ramans Harbo-rough S, Zhang Y, Ljung K, Callis J, Almqvist F, Kepinski S, Estelle M, Pauwels L, Robert S (2019). Selective auxin agonists induce specific AUX/IAA protein degradation to modulate plant development. Proc Natl Acad Sci USA 116, 6463-6472. |
| [40] | Wang RH, Estelle M (2014). Diversity and specificity: auxin perception and signaling through the TIR1/AFB pathway. Curr Opin Plant Biol 21, 51-58. |
| [41] | Yu ZP, Zhang F, Friml J, Ding ZJ (2022). Auxin signaling: research advances over the past 30 years. J Integr Plant Biol 64, 371-392. |
| [42] | Zhong SW, Shi H, Xue C, Wei N, Guo HW, Deng XW (2014). Ethylene-orchestrated circuitry coordinates a seedling’s response to soil cover and etiolated growth. Proc Natl Acad Sci USA 111, 3913-3920. |
/
| 〈 |
|
〉 |