

根癌农杆菌介导万寿菊遗传转化体系的建立
收稿日期: 2022-07-02
录用日期: 2022-12-02
网络出版日期: 2022-12-23
基金资助
国家自然科学基金(32172616);特色花卉生物育种湖北省工程研究中心开放课题(2022ZD007)
Establishment of Agrobacterium tumefaciens-mediated Genetic Transformation System of Marigold (Tagetes erecta)
Received date: 2022-07-02
Accepted date: 2022-12-02
Online published: 2022-12-23
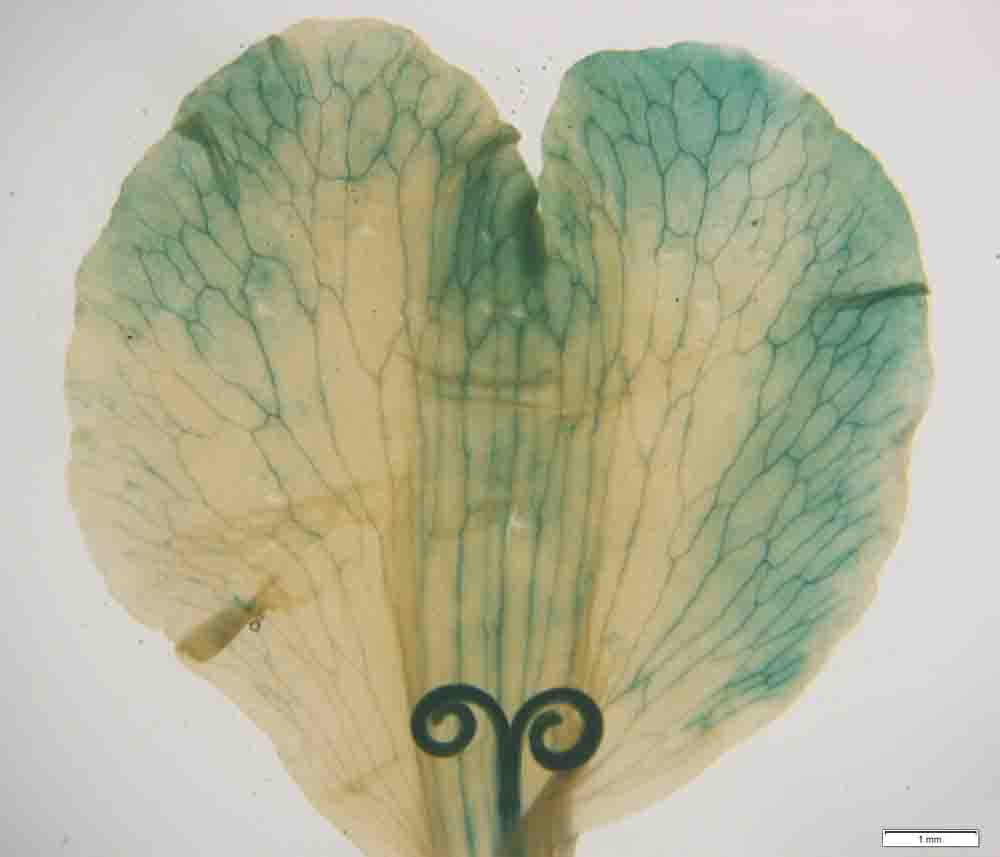
余晓敏 , 王亚琴 , 刘雨菡 , 易庆平 , 程文翰 , 朱钰 , 段枫 , 张莉雪 , 何燕红 . 根癌农杆菌介导万寿菊遗传转化体系的建立[J]. 植物学报, 2023 , 58(5) : 760 -769 . DOI: 10.11983/CBB22141

Key words: Agrobacterium tumefaciens; genetic transformation; leaflet; marigold
| [1] | 陈利文, 唐楠 (2021). 不同品种色素万寿菊主要农艺性状评价. 安徽农业科学 49(4), 53-55. |
| [2] | 付洪冰, 崔崇士, 赵曦, 刘琦 (2010). 农杆菌介导南瓜遗传转化体系的建立. 植物学报 45, 472-478. |
| [3] | 符勇耀, 杨利平, 郑开敏, 徐文姬 (2021). 药用万寿菊多倍体的诱导与特征分析. 热带作物学报 42, 1318-1325. |
| [4] | 郭彩珍 (2021). 响应面法优化紫丁香愈伤组织诱导条件及防褐化研究. 种子 40(8), 141-145, 148. |
| [5] | 梁艳, 赵雪莹, 白雪, 刘德强, 张妍, 潘朋 (2021). PVP处理对黑皮油松外植体酚类物质形成及酶活性的影响. 林业科学 57(10), 166-174. |
| [6] | 刘翰升, 赵春莉, 刘玥, 国伟强 (2019). 镉胁迫对万寿菊属植物幼苗生理及富集的影响. 福建农业学报 34, 1221-1227. |
| [7] | 刘香利, 赵惠贤, 郭蔼光 (2011). 农杆菌介导的小麦遗传转化研究进展. 安徽农业科学 39, 19065-19066. |
| [8] | 王关林, 方宏筠 (2002). 植物基因工程(第2版). 北京: 科学出版社. pp. 393. |
| [9] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. |
| [10] | 杨帆 (2011). 色素万寿菊psy双边界载体及遗传转化体系建立和SSR-PCR体系的优化. 硕士论文. 上海: 上海交通大学. pp. 14-23. |
| [11] | 曾益, 李婧怡, 周明康, 曹栋才, 娄琳琳, 何恒, 何梦雪, 邹俊杰, 殷中琼 (2021). 万寿菊茎叶醇提物的镇痛抗炎活性研究. 四川农业大学学报 39, 451-458. |
| [12] | 张海霞, 张少英, 付增娟 (2013). 乙酰丁香酮对甜菜遗传转化的影响. 广东农业科学 40(22), 22-24. |
| [13] | 张嫔 (2012). 万寿菊属植物染色体核型分析及万寿菊psy基因遗传转化体系影响因素的研究. 硕士论文. 上海: 上海交通大学. pp. 36-47. |
| [14] | Amoah BK, Wu H, Sparks C, Jones HD (2001). Factors influencing Agrobacterium-mediated transient expression of uidA in wheat inflorescence tissue. J Exp Bot 52, 1135-1142. |
| [15] | Cervera M, López MM, Navarro L, Pe?a L (1998). Virulence and supervirulence of Agrobacterium tumefaciensin woody fruit plants. Physiol Mol Plant Pathol 52, 67-78. |
| [16] | Chintakovid W, Visoottiviseth P, Khokiattiwong S, Lauengsuchonkul S (2008). Potential of the hybrid marigolds for arsenic phytoremediation and income generation of remediators in Ron Phibun District, Thailand. Chemosphere 70, 1532-1537. |
| [17] | Chitrakar B, Zhang M, Bhandari B (2019). Edible flowers with the common name “Marigold”: their therapeutic values and processing. Trends Food Sci Technol 89, 76-87. |
| [18] | Godoy-Hernández G, Berzunza EA, Concha LC, Miranda- Ham MDL (2006). Agrobacterium-mediated transient transformation of marigold (Tagetes erecta). Plant Cell Tissue Organ Cult 84, 365-368. |
| [19] | Gupta V, Rahman LU (2015). An efficient plant regeneration and Agrobacterium-mediated genetic transformation of Tagetes erecta. Protoplasma 252, 1061-1070. |
| [20] | Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901-3907. |
| [21] | Lacatusu I, Badea G, Popescu M, Bordei N, Istrati D, Moldovan L, Seciu AM, Panteli MI, Rasit I, Badea N (2017). Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind Crops Prod 109, 141-150. |
| [22] | Maleki SS, Mohammadi K, Ji KS (2018). Study on factors influencing transformation efficiency in Pinus massoniana using Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult 133, 437-445. |
| [23] | Manivannan A, Narasegowda S, Prakash T (2021). Comparative study on color coordinates, phenolics, flavonoids, carotenoids, and antioxidant potential of marigold (Tagetes sp.) with diverse colored petals. J Food Meas Charact 15, 4343-4353. |
| [24] | Mir RA, Argal S, Ahanger MA, Tomar NS, Agarwal RM (2022). Variation in phenolic compounds, antioxidant activity and osmotica of different cultivars of Tagetes erecta L. at different growth stages and effect of its leachates on germination and growth of wheat (Triticum aestivum L.). J Plant Growth Regul 41, 907-921. |
| [25] | Nuoendagula, Narushima M, Uesugi M, Murai Y, Katayama Y, Iimura Y, Kajita S (2017). In vitro regeneration and Agrobacterium-mediated transformation of male- sterile marigold (Tagetes erecta L.). Plant Biotechnol (Tokyo) 34, 125-129. |
| [26] | Sessitsch A, Hardoim P, D?ring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M, Hurek T, Sarkar A, Bodrossy L, Van Overbeek L, Brar D, Van Elsas JD, Reinhold-Hurek B (2012). Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant Microbe Interact 25, 28-36. |
| [27] | Shabbir M, Rather LJ, Mohammad F (2018). Economically viable UV-protective and antioxidant finishing of wool fabric dyed with Tagetes erecta flower extract: valorization of marigold. Ind Crops Prod 119, 277-282. |
| [28] | Stachel SE, Messens E, Van Montagu M, Zambryski P (1985). Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318, 624-629. |
| [29] | Vanegas PE, Valdez-Morales M, Valverde ME, Cruz-Hernández A, Paredes-López O (2006). Particle bombardment, a method for gene transfer in marigold. Plant Cell Tissue Organ Cult 84, 359-363. |
/
| 〈 |
|
〉 |