

濒危植物大别山五针松根际细菌群落特征与功能分析
收稿日期: 2022-03-07
修回日期: 2022-05-30
网络出版日期: 2022-05-30
基金资助
安徽省自然科学青年基金(2108085QC138);安徽省重点研究与开发计划(202104f06020024);国家自然科学基金(31800316);安徽省重点实验室开放基金(Wy2021001);安徽省重点实验室开放基金(Wz2021002);安徽省重点实验室开放基金(Wsz202204);安徽省重点实验室开放基金(Wsz202207);安徽省重点实验室开放基金(Wy202206);安徽高校自然科学研究项目(KJ2021A0647);国家级创新创业项目(202110732044X);安徽省大学生创新创业训练计划(S202110372104X);安徽省大学生创新创业训练计划(S202110372107);2021年度安庆师范大学研究生线上课程项目(2021aqnuxskc02);研究生教育教学改革项目(2021aqnujyxm64);“敬敷育英”创新创业引领计划和安庆师范大学教研项目(2019aqnujyzc127)
Characteristics and Function Analysis of Rhizosphere Bacterial Community of Endangered Plant Pinus dabeshanensis
Received date: 2022-03-07
Revised date: 2022-05-30
Online published: 2022-05-30
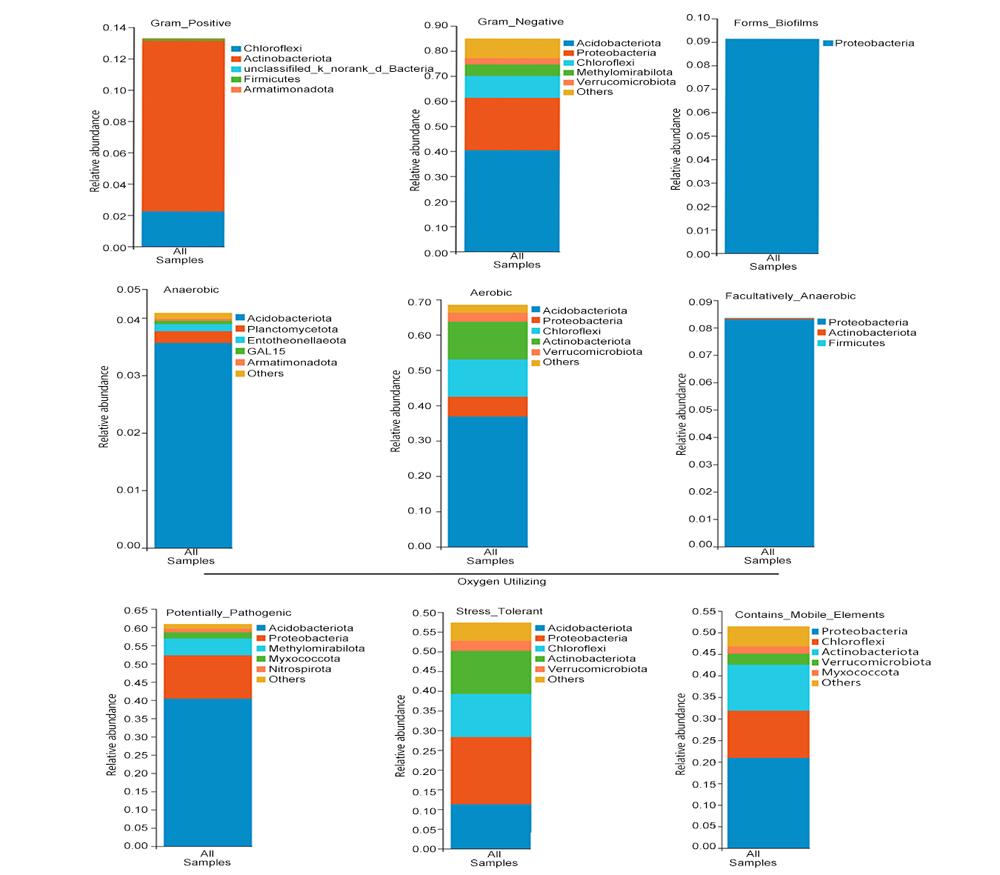
植物根际微生物群落对植物健康生长有重要影响, 每种植物根际都有其特定的微生物群落。大别山五针松(Pinus dabeshanensis)被国际自然保护联盟列为濒危物种, 具有重要研究价值。该研究采用16S rRNA高通量测序技术与生物信息学方法, 对濒危植物大别山五针松根际细菌群落特征与功能进行分析。结果表明, 大别山五针松根际微生物的主要种类为变形菌门、放线菌门、酸杆菌门和疣微菌门。网络分析表明, 大别山五针松根际细菌类群存在显著相关性, 其中Bryobacter属、Bradyrhizobium属和未定义的TK10属是互作网络中的重要节点。PICRUSt1功能预测表明其微生物组功能主要为氨基酸运输和代谢、细胞壁/膜/膜生物发生以及能量产生和转换。FAPROTAX功能预测表明, 大别山五针松根际富含的优势菌群具有丰富的化学异养、纤维素水解、需氧化学异养和固氮功能, 其对植物生长发育具有重要作用。研究结果可为培育健康的大别山五针松根际微生物菌群及微生物资源的开发利用提供重要依据。
石水琴 , 秦华光 , 张静静 , 韩钰 , 余淏 , 彭怡宁 , 杨邵 , 汪嘉怡 , 何光宇 , 岂泽华 , 吴文杰 , 朱星雨 , 饶玉春 , 穆丹 . 濒危植物大别山五针松根际细菌群落特征与功能分析[J]. 植物学报, 2022 , 57(4) : 457 -467 . DOI: 10.11983/CBB22040
Plant rhizosphere microbial community markedly impact on the healthy growth of plants, and each plant rhizosphere has its own specific microbial community. Pinus dabeshanensis was listed as an endangered species by the International Union for Conservation of Nature, and it is very important for scientific research. In order to explore the characteristics and functions of rhizosphere bacterial community of this endangered species, high-throughput sequencing and bioinformatics analysis on bacterial 16S rRNA were used. The results showed that the major species of rhizosphere microorganisms of P. dabeshanensis were Proteobacteria, Actinobacteriota, Acidobacteriota and Verrucomicrobiota. Network analysis showed that there were significant correlations among rhizosphere bacterial groups of P. dabeshanensis, Bryobacter, Bradyrhizobium and an unidentified genus TK10 were important nodes in the network. PICRUSt1 function prediction indicated that the microbiome functions mainly involved with amino acid transport and metabolism, cell wall/membrane/membrane biogenesis, energy production and conversion. The functional prediction of FAPROTAX showed that the dominant flora in the rhizosphere of P. dabeshanensis had functions enriched in such processes as chemical heterotrophic, cellulose hydrolysis, aerobic chemical heterotrophic and nitrogen fixation, which plays an important role in plant growth and development. The results of this study provide a research basis for cultivating healthy rhizosphere microbial flora for P. dabeshanensis, and for the development and utilization of microbial resources for a better growth of plants.

| [1] | 宋健, 张海剑, 刘莉, 杜立新, 柳健虎, 曹伟平 (2020). 高通量测序分析高温覆膜对韭菜根际微生物多样性的影响. 中国生物防治学报 36, 938-945. |
| [2] | 滕泽栋, 李敏, 朱静, 宋明阳 (2017). 野鸭湖湿地芦苇根际微生物多样性与磷素形态关系. 环境科学 38, 4589-4597. |
| [3] | 王孝林, 王二涛 (2019). 根际微生物促进水稻氮利用的机制. 植物学报 54, 285-287. |
| [4] | 王亚茹, 田宝玉, 张碧尧, 范競文, 戈峰, 王国红 (2021). 饵料蛋白水平对德国小蠊肠道细菌群落的影响. 生态学报 41, 5495-5505. |
| [5] | Agler MT, Ruhe J, Kroll S, Morhenn C, Kim ST, Weigel D, Kemen EM (2016). Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol 14, e1002352. |
| [6] | Babalola OO, Emmanuel OC, Adeleke BS, Odelade KA, Nwachukwu BC, Ayiti OE, Adegboyega TT, Igiehon NO (2021). Rhizosphere microbiome cooperations: strategies for sustainable crop production. Curr Microbiol 78, 1069-1085. |
| [7] | Bano S, Wu XG, Zhang XJ (2021). Towards sustainable agriculture: rhizosphere microbiome engineering. Appl Microbiol Biotechnol 105, 7141-7160. |
| [8] | Berendsen RL, Pieterse CMJ, Bakker PAHM (2012). The rhizosphere microbiome and plant health. Trends Plant Sci 17, 478-486. |
| [9] | Bulgarelli D, Garrido-Oter R, Münch PC, Weiman A, Dröge J, Pan Y, McHardy AC, Schulze-Lefert P (2015). Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17, 392-403. |
| [10] | Campbell BJ, Engel AS, Porter ML, Takai K (2006). The versatile ε-proteobacteria: key players in sulphidic habitats. Nat Rev Microbiol 4, 458-468. |
| [11] | Dai ZM, Su WQ, Chen HH, Barberán A, Zhao HC, Yu MJ, Yu L, Brookes PC, Schadt CW, Chang SX, Xu JM (2018). Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob Chang Biol 24, 3452-3461. |
| [12] | Delgado-Baquerizo M, Grinyer J, Reich PB, Singh BK, Allen E (2016). Relative importance of soil properties and microbial community for soil functionality: insights from a microbial swap experiment. Funct Ecol 30, 1862-1873. |
| [13] | Durán P, Thiergart T, Garrido-Oter R, Agler M, Kemen E, Schulze-Lefert P, Hacquard S (2018). Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 175, 973-983. |
| [14] | Edgar RC (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460-2461. |
| [15] | Eren AM, Vineis JH, Morrison HG, Sogin ML (2013). A filtering method to generate high quality short reads using illumina paired-end technology. PLoS One 8, e66643. |
| [16] | Fierer N (2017). Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15, 579-590. |
| [17] | Fu LK, Chin CM (1992). China Plant Red Data Book:Rare and Endangered Plants. Beijing: Science Press. pp. 46-47. |
| [18] | Jacoby RP, Chen L, Schwier M, Koprivova A, Kopriva S (2020). Recent advances in the role of plant metabolites in shaping the root microbiome. F1000Res 9, 151. |
| [19] | Keswani C, Prakash O, Bharti N, Vilchez JI, Sansinenea E, Lally RD, Borriss R, Singh SP, Gupta VK, Fraceto LF, de Lima R, Singh HB (2019). Re-addressing the biosafety issues of plant growth promoting rizobacteria. Sci Total Environ 690, 841-852. |
| [20] | Li YB, Li Q, Chen SF (2021). Diazotroph Paenibacillus triticisoli BJ-18 drives the variation in bacterial, diazotrophic and fungal communities in the rhizosphere and root/shoot endosphere of maize. Int J Mol Sci 22, 1460. |
| [21] | Ling N, Song Y, Raza W, Huang QW, Guo SW, Shen QR (2015). The response of root-associated bacterial commu- nity to the grafting of watermelon. Plant Soil 391, 253-264. |
| [22] | Lucas G, Synge H (1978). The IUCN Plant Red Data Book: Comprising Red Data Sheets on Two Hundred-fifty Selected Plants Threatened on a World Scale. Gland: IUCN. pp. 365-366. |
| [23] | Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Geh- ring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL (2012). Defining the core Arabidopsis thaliana root microbiome. Nature 488, 86-90. |
| [24] | Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014). Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J 8, 1577-1587. |
| [25] | Nihorimbere V, Ongena M, Smargiassi M, Thonart P (2011). Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol Agron Soc Environ 15, 327-337. |
| [26] | Noman M, Ahmed T, Ijaz U, Shahid M, Azizullah, Li DY, Manzoor I, Song FM (2021). Plant-microbiome crosstalk: dawning from composition and assembly of microbial community to improvement of disease resilience in plants. Int J Mol Sci 22, 6852. |
| [27] | Qu Q, Zhang ZY, Peijnenburg WJGM, Liu WY, Lu T, Hu BL, Chen JM, Chen J, Lin ZF, Qian HF (2020). Rhizosphere microbiome assembly and its impact on plant growth. J Agric Food Chem 68, 5024-5038. |
| [28] | Reinhold-Hurek B, Bϋnger W, Burbano CS, Sabale M, Hurek T (2015). Roots shaping their microbiome: global hotspots for microbial activity. Annu Rev Phytopathol 53, 403-424. |
| [29] | Sarkar J, Chakraborty B, Chakraborty U (2018). Plant growth promoting rhizobacteria protect wheat plants against temperature stress through antioxidant signaling and reducing chloroplast and membrane injury. J Plant Growth Regul 37, 1396-1412. |
| [30] | Tang HM, Xiao XP, Li C, Pan XC, Cheng KK, Li WY, Wang K (2020). Microbial carbon source utilization in rice rhizosphere and nonrhizosphere soils with short-term manure N input rate in paddy field. Sci Rep 10, 6487. |
| [31] | Tian L, Chang JJ, Shi SH, Ji L, Zhang JF, Sun Y, Li XJ, Li XJ, Xie HW, Cai YH, Chen DZ, Wang JL, van Veen JA, Kuramae EE, Tran LSP, Tian CJ (2022). Comparison of methane metabolism in the rhizomicrobiomes of wild and related cultivated rice accessions reveals a strong impact of crop domestication. Sci Total Environ 803, 150131. |
| [32] | Tiepo AN, Hertel MF, Rocha SS, Calzavara AK, De Oliveira ALM, Pimenta JA, Oliveira HC, Bianchini E, Stolf-Moreira R (2018). Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol Biochem 130, 277-288. |
| [33] | Trivedi P, Leach JE, Tringe SG, Sa TM, Singh BK (2021). Plant-microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18, 607-621. |
| [34] | Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Xie G, Haft DH, Sait M, Badger J, Barabote RD, Bradley B, Brettin TS, Brinkac LM, Bruce D, Creasy T, Daugherty SC, Davidsen TM, DeBoy RT, Detter JC, Dodson RJ, Durkin AS, Ganapathy A, Gwinn-Giglio M, Han CS, Khouri H, Kiss H, Kothari SP, Madupu R, Nelson KE, Nelson WC, Paulsen I, Penn K, Ren QH, Rosovitz MJ, Selengut JD, Shrivastava S, Sullivan SA, Tapia R, Thompson LS, Watkins KL, Yang Q, Yu CH, Zafar N, Zhou LW, Kuske CR (2009). Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microbiol 75, 2046-2056. |
| [35] | Warrad M, Hassan YM, Mohamed MSM, Hagagy N, Al- Maghrabi OA, Selim S, Saleh AM, AbdElgawad H (2020). A bioactive fraction from Streptomyces sp. enhances maize tolerance against drought stress. J Microbiol Biotechnol 30, 1156-1168. |
| [36] | Xu J, Zhang YZ, Zhang PF, Trivedi P, Riera N, Wang YY, Liu X, Fan GY, Tang JL, Coletta-Filho HD, Cubero J, Deng XL, Ancona V, Lu ZJ, Zhong BL, Roper MC, Capote N, Catara V, Pietersen G, Vernière C, Al-Sadi AM, Li L, Yang F, Xu X, Wang J, Yang HM, Jin T, Wang N (2018). The structure and function of the global citrus rhizosphere microbiome. Nat Commun 9, 4894. |
| [37] | Zhu ZK, Ge TD, Hu YJ, Zhou P, Wang TT, Shibistova O, Guggenberger G, Su YR, Wu JS (2017). Fate of rice shoot and root residues, rhizodeposits, and microbial assimilated carbon in paddy soil-part 2: turnover and microbial utilization. Plant Soil 416, 243-257. |
| [38] | Zhu ZK, Ge TD, Liu SL, Hu YJ, Ye RZ, Xiao ML, Tong CL, Kuzyakov Y, Wu JS (2018). Rice rhizodeposits affect organic matter priming in paddy soil: the role of N fertilization and plant growth for enzyme activities, CO2 and CH4 emissions. Soil Biol Biochem 116, 369-377. |
/
| 〈 |
|
〉 |