

叶脉结构与功能及其对叶片经济谱的影响
收稿日期: 2021-11-23
录用日期: 2022-03-18
网络出版日期: 2022-03-18
基金资助
国家重大研发计划(2017YFC0505105);第二次青藏高原综合考察研究(2019QZKK0301);国家科技基础资源调查专项(2019FY202300)
The Structure and Function of Leaf Veins and Their Influence on Leaf Economic Spectrum
Received date: 2021-11-23
Accepted date: 2022-03-18
Online published: 2022-03-18
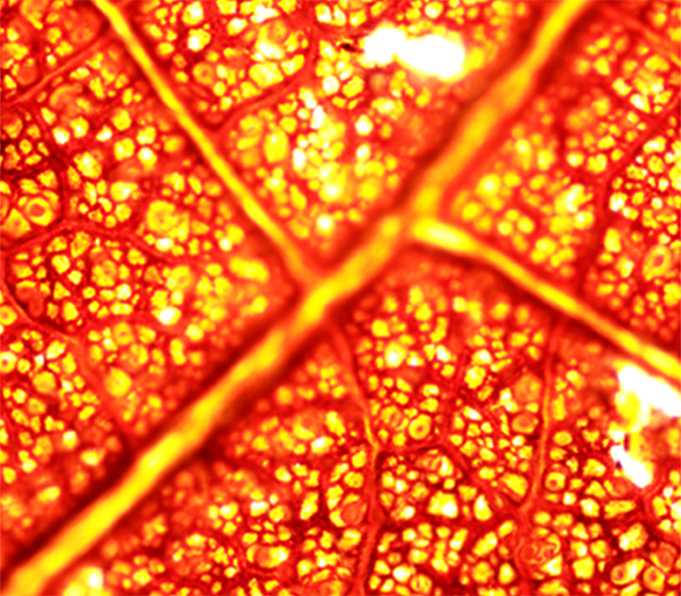
叶脉由贯穿于叶肉内部的维管组织及其外围机械组织构成, 多样化的脉序及网络结构使叶脉系统发生变异和功能分化。该文综述了叶脉系统结构与功能的最新研究进展。通过聚焦叶脉分级系统的结构与功能及其在叶片经济谱(LES)中的重要性, 解释叶脉性状与其它叶片功能性状之间的关系及机制。不同等级叶脉在机械支撑与水分运输方面存在功能分化, 其中1-3级粗脉在维持叶片形状和叶表面积以及物理支撑方面发挥重要作用, 有利于维持叶片最大受光面积; 4级及以上细脉具有水分调节功能, 它们与气孔相互协调, 影响叶片水分运输、蒸腾散热和光合作用速率。叶片生长过程与叶脉发育的动态变化模式决定叶脉密度, 并影响叶脉密度与叶片大小之间的关系: 叶面积与粗脉密度呈显著负相关, 与粗脉直径呈显著正相关, 而与细脉密度无关。与叶脉性状相关的叶片经济谱框架模型预测, 叶脉密度较高的叶片寿命短、比叶重较小, 叶片最大碳同化速率、代谢速率以及资源获取策略潜力较高。
吴一苓, 李芳兰, 胡慧 . 叶脉结构与功能及其对叶片经济谱的影响[J]. 植物学报, 2022 , 57(3) : 388 -398 . DOI: 10.11983/CBB21203
Vascular tissues inside the mesophyll and peripheral mechanical tissues constitute the veins. The diverse orders and network structures of veins contribute to their functional diversification and differentiation. In this review, we summarized the research progresses on the structure and function of the leaf vein system. We reviewed three aspects of veins and critically evaluated the characteristics of the leaf vein hierarchy system and its important role in leaf economic spectrum (LES), and explained the mechanisms linking vein traits and other functional traits of the leaf. Leaf veins of different orders show obvious functional differentiation in terms of hydraulic conduction and mechanical support. Among them, the first three orders of veins (major veins) play a major role in maintaining leaf shape, leaf surface area and physical support, and which is conductive to the growth of leaves with the largest light-receiving area. The higher order veins (minor veins) have the function of water regulation, and their coordination with the stomata determines the rate of leaf water transport, transpiration and photosynthesis. The patterns of dynamic variation in leaf spread and leaf vein development explain the relationship between vein density and leaf size. Leaf surface area is negatively correlated with the density of main veins and positively correlated with the diameter of main veins, but independent of the density of minor veins. The framework model of LES linking with vein traits predicts that leaves with higher vein density have short lifespan and smaller leaf mass per area, which explains the better leaf carbon assimilation rate, metabolism rate and resource acquisition strategy with higher leaf vein density.

| [1] | 刘晓娟, 马克平 (2015). 植物功能性状研究进展. 中国科学: 生命科学 45, 325-339. |
| [2] | 罗丽莹, 陈楠, 王云龙, 王光军 (2021). 闽楠叶形态与叶脉网络性状关系对城市生长环境的响应. 生态学报 41, 7838- 7847. |
| [3] | 宋丽清, 胡春梅, 侯喜林, 石雷, 刘立安, 杨景成, 姜闯道 (2015). 高粱、紫苏叶脉密度与光合特性的关系. 植物学报 50, 100-106. |
| [4] | 孙素静, 李芳兰, 包维楷 (2015). 叶脉网络系统的构建和系统学意义研究进展. 热带亚热带植物学报 23, 353-360. |
| [5] | 徐龙, 贺鹏程, 张统, 刘慧, 叶清 (2020). 不同原生境的6种棕榈科植物叶片水力性状的对比研究. 热带亚热带植物学报 28, 472-478. |
| [6] | 姚广前, 魏阳, 毕敏慧, 聂争飞, 方向文 (2018). 干旱胁迫下4种锦鸡儿属植物叶脉密度与最低水势关系. 中国沙漠 38, 1252-1258. |
| [7] | 朱济友, 徐程扬, 刘亚培, 李金航, 黄涛, 覃国铭, 崔哲浩 (2019). 基于遥感的植物叶脉功能性状计算及其生态学意义. 生态科学 38, 209-216. |
| [8] | Amakawa T (1981). Studies on the character of leaves for distinguishing tree-species in the field, 2. The venation of leaves of dicotyledons. Bulletin of Nakamura Gakuen University and Nakamura Gakuen Junior College 14, 13- 22. |
| [9] | Banavar JR, Maritan A, Rinaldo A (1999). Size and form in efficient transportation networks. Nature 399, 130-132. |
| [10] | Beerling DJ, Franks PJ (2010). The hidden cost of transpiration. Nature 464, 495-496. |
| [11] | Blonder B, Baldwin BG, Enquist BJ, Robichaux RH (2016). Variation and macroevolution in leaf functional traits in the Hawaiian silversword alliance (Asteraceae). J Ecol 104, 219-228. |
| [12] | Blonder B, Both S, Jodra M, Xu H, Fricker M, Matos IS, Majalap N, Burslem DFRP, Teh YA, Malhi Y (2020). Linking functional traits to multiscale statistics of leaf venation networks. New Phytol 228, 1796-1810. |
| [13] | Blonder B, Enquist BJ (2014). Inferring climate from angiosperm leaf venation networks. New Phytol 204, 116-126. |
| [14] | Blonder B, Vasseur F, Violle C, Shipley B, Enquist BJ, Vile D (2015). Testing models for the leaf economics spectrum with leaf and whole-plant traits in Arabidopsis thaliana. AoB Plants 7, plv049. |
| [15] | Blonder B, Violle C, Bentley LP, Enquist BJ (2011). Venation networks and the origin of the leaf economics spectrum. Ecol Lett 14, 91-100. |
| [16] | Blonder B, Violle C, Bentley LP, Enquist BJ (2014). Inclusion of vein traits improves predictive power for the leaf economic spectrum: a response to Sack et al. (2013). J Exp Bot 65, 5109-5114. |
| [17] | Blonder B, Violle C, Enquist BJ (2013). Assessing the causes and scales of the leaf economics spectrum using venation networks in Populu tremuloides. J Ecol 101, 981-989. |
| [18] | Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA (2009). Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc Biol Sci 276, 1771- 1776. |
| [19] | Brodribb TJ, Feild TS (2010). Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett 13, 175-183. |
| [20] | Brodribb TJ, Feild TS, Jordan GJ (2007). Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144, 1890-1898. |
| [21] | Brodribb TJ, Feild TS, Sack L (2010). Viewing leaf structure and evolution from a hydraulic perspective. Funct Plant Biol 37, 488-498. |
| [22] | Brodribb TJ, Jordan GJ (2011). Water supply and demand remain balanced during leaf acclimation of Nothofagus cunninghamii trees. New Phytol 192, 437-448. |
| [23] | Chen XP, Sun J, Wang MT, Lyu M, Niklas KJ, Michaletz ST, Zhong QL, Cheng DL (2020). The leaf economics spectrum constrains phenotypic plasticity across a light gradient. Front Plan Sci 11, 735. |
| [24] | Choat B, Lahr EC, Melcher PJ, Zwieniecki MA, Holbrook NM (2005). The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant Cell Environ 28, 1082- 1089. |
| [25] | Coley PD (1983). Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr 53, 209-234. |
| [26] | Dodds PS (2010). Optimal form of branching supply and collection networks. Phys Rev Lett 104, 048702. |
| [27] | Dow GJ, Berry JA, Bergmann DC (2013). The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsi thaliana. New Phytol 201, 1205-1217. |
| [28] | Dunbar-Co S, Sporck MJ, Sack L (2009). Leaf trait diversification and design in seven rare taxa of the Hawaiian plantago radiation. Int J Plant Sci 170, 61-75. |
| [29] | Durand M (2006). Architecture of optimal transport networks. Phys Rev E 73, 016116. |
| [30] | Durand M (2007). Structure of optimal transport networks subject to a global constraint. Phys Rev Lett 98, 088701. |
| [31] | Fajardo A, Siefert A (2018). Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 99, 1024-1030. |
| [32] | Fiorin L, Brodribb TJ, Anfodillo T (2016). Transport efficiency through uniformity: organization of veins and stomata in angiosperm leaves. New Phytol 209, 216-227. |
| [33] | Hickey LJ (1973). Classification of the architecture of dicotyledonous leaves. Am J Bot 60, 17-33. |
| [34] | Hua L, He P, Goldstein G, Liu H, Yin D, Zhu S, Ye Q (2020). Linking vein properties to leaf biomechanics across 58 woody species from a subtropical forest. Plant Biol J 22, 212-220. |
| [35] | Ji WL, LaZerte SE, Waterway MJ, Lechowicz MJ (2020). Functional ecology of congeneric variation in the leaf economics spectrum. New Phytol 225, 196-208. |
| [36] | John GP, Scoffoni C, Buckley TN, Villar R, Poorter H, Sack L (2017). The anatomical and compositional basis of leaf mass per area. Ecol Lett 20, 412-425. |
| [37] | Jordan GJ, Brodribb TJ, Blackman CJ, Weston PH (2013). Climate drives vein anatomy in Proteaceae. Am J Bot 100, 1483-1493. |
| [38] | Kang J, Dengler N (2004). Vein pattern development in adult leaves of Arabidopsi thaliana. Int J Plant Sci 165, 231-242. |
| [39] | Kang J, Mizukami Y, Wang H, Fowke L, Dengler NG (2007). Modification of cell proliferation patterns alters leaf vein architecture in Arabidopsi thaliana. Planta 226, 1207- 1218. |
| [40] | Katifori E, Szöllősi GJ, Magnasco MO (2010). Damage and fluctuations induce loops in optimal transport networks. Phys Rev Lett 104, 048704. |
| [41] | Kawai K, Okada N (2016). How are leaf mechanical properties and water-use traits coordinated by vein traits? A case study in Fagaceae. Funct Ecol 30, 527-536. |
| [42] | Kawai K, Okada N (2019). Leaf vascular architecture in temperate dicotyledons: correlations and link to functional traits. Planta 251, 17. |
| [43] | Kitajima K, Poorter L (2010). Tissue-level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytol 186, 708-721. |
| [44] | Li FL, McCulloh KA, Sun SJ, Bao WK (2018). Linking leaf hydraulic properties, photosynthetic rates, and leaf lifespan in xerophytic species: a test of global hypotheses. Am J Bot 105, 1858-1868. |
| [45] | Lloyd J, Bloomfield K, Domingues TF, Farquhar GD (2013). Photosynthetically relevant foliar traits correlating better on a mass vs an area basis: of ecophysiological relevance or just a case of mathematical imperatives and statistical quicksand? New Phytol 199, 311-321. |
| [46] | Luo LY, Chen N, Wang YL, Wang GJ (2021). Response of leaf morphology and vein network traits of Phoebe bournei to urban growth environment. Acta Ecol Sin 41, 7838- 7847. |
| [47] | Martin AR, Isaac ME (2021). The leaf economics spectrum’s morning coffee: plant size-dependent changes in leaf traits and reproductive onset in a perennial tree crop. Ann Bot 127, 483-493. |
| [48] | McKown AD, Cochard H, Sack L (2010). Decoding leaf hydraulics with a spatially explicit model: principles of venation architecture and implications for its evolution. Am Nat 175, 447-460. |
| [49] | Meziane D, Shipley B (2001). Direct and indirect relationships between specific leaf area, leaf nitrogen and leaf gas exchange. Effects of irradiance and nutrient supply. Ann Bot 88, 915-927. |
| [50] | Murphy MRC, Jordan GJ, Brodribb TJ (2012). Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ 35, 1407-1418. |
| [51] | Nardini A, Pedà G, La Rocca N (2012). Trade-offs between leaf hydraulic capacity and drought vulnerability: morpho-anatomical bases, carbon costs and ecological consequences. New Phytol 196, 788-798. |
| [52] | Nardini A, Ramani M, Gortan E, Salleo S (2008). Vein recovery from embolism occurs under negative pressure in leaves of sunflower (Helianthus annuus). Physiol Plant 133, 755-764. |
| [53] | Navas ML, Ducout B, Roumet C, Richarte J, Garnier J, Garnier E (2003). Leaf life span, dynamics and construction cost of species from Mediterranean old-fields differing in successional status. New Phytol 159, 213-228. |
| [54] | Niinemets Ü (1999). Research review. Components of leaf dry mass per area-thickness and density-alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144, 35-47. |
| [55] | Niinemets Ü, Portsmuth A, Tobias M (2007). Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: a neglected source of leaf physiological differentiation? Funct Ecol 21, 28-40. |
| [56] | Niinemets Ü, Sack L (2006). Structural determinants of leaf light-harvesting capacity and photosynthetic potentials. In: Esser K, Lüttge U, Beyschlag W, Murata J, eds. Progress in Botany. Berlin, Heidelberg: Springer. pp. 385-419. |
| [57] | Niklas KJ (1999). A mechanical perspective on foliage leaf form and function. New Phytol 143, 19-31. |
| [58] | Noblin X, Mahadevan L, Coomaraswamy IA, Weitz DA, Holbrook NM, Zwieniecki MA (2008). Optimal vein density in artificial and real leaves. Proc Natl Acad Sci USA 105, 9140-9144. |
| [59] | Onoda Y, Westoby M, Adler PB, Choong AMF, Clissold FJ, Cornelissen JHC, Díaz S, Dominy NJ, Elgart A, Enrico L, Fine PVA, Howard JJ, Jalili A, Kitajima K, Kurokawa H, McArthur C, Lucas PW, Markesteijn L, Pérez-Harguindeguy N, Poorter L, Richards L, Santiago LS, Sosinski EE Jr, Van Bael SA, Warton DI, Wright IJ, Wright SJ, Yamashita N (2011). Global patterns of leaf mechanical properties. Ecol Lett 14, 301-312. |
| [60] | Osnas JLD, Lichstein JW, Reich PB, Pacala SW (2013). Global leaf trait relationships: mass, area, and the leaf economics spectrum. Science 340, 741-744. |
| [61] | Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182, 565-588. |
| [62] | Price CA, Wing S, Weitz JS (2012). Scaling and structure of dicotyledonous leaf venation networks. Ecol Lett 15, 87- 95. |
| [63] | Read J, Sanson GD (2003). Characterizing sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytol 160, 81-99. |
| [64] | Read J, Stokes A (2006). Plant biomechanics in an ecological context. Am J Bot 93, 1546-1565. |
| [65] | Reich PB, Walters MB, Ellsworth DS (1997). From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94, 13730-13734. |
| [66] | Roderick ML, Berry SL, Noble IR, Farquhar GD (1999). A theoretical approach to linking the composition and morphology with the function of leaves. Funct Ecol 13, 683- 695. |
| [67] | Rolland-Lagan AG, Amin M, Pakulska M (2009). Quantifying leaf venation patterns: two-dimensional maps. J Plant 57, 195-205. |
| [68] | Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H (2001). Evolution and function of leaf venation architecture: a review. Ann Bot 87, 553-566. |
| [69] | Sack L, Cowan PD, Jaikumar N, Holbrook NM (2003). The ‘hydrology’ of leaves: coordination of structure and function in temperate woody species. Plant Cell Environ 26, 1343-1356. |
| [70] | Sack L, Holbrook NM (2006). Leaf hydraulics. Annu Rev Plant Biol 57, 361-381. |
| [71] | Sack L, Scoffoni C (2013). Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol 198, 983-1000. |
| [72] | Sack L, Scoffoni C, John GP, Poorter H, Mason CM, Mendez-Alonzo R, Donovan LA (2013). How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J Exp Bot 64, 4053-4080. |
| [73] | Sack L, Scoffoni C, McKown AD, Frole K, Rawls M, Havran JC, Tran H, Tran T (2012). Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat Commun 3, 837. |
| [74] | Sack L, Streeter CM, Holbrook NM (2004). Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiol 134, 1824-1833. |
| [75] | Schoch PG, Zinsou C, Sibi M (1980). Dependence of the stomatal index on environmental factors during stomatal differentiation in leaves of Vigna sinensis L. 1. Effect of light intensity. J Exp Bot 31, 1211-1216. |
| [76] | Scoffoni C, Rawls M, McKown A, Cochard H, Sack L (2011). Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol 156, 832-843. |
| [77] | Shipley B, Lechowicz MJ, Wright I, Reich PB (2006). Fundamental trade-offs generating the worldwide leaf eco- nomics spectrum. Ecology 87, 535-541. |
| [78] | Turgeon R (2006). Phloem loading: how leaves gain their independence. BioScience 56, 15-24. |
| [79] | Tyree MT, Sobrado M, Stratton LJ, Becker P (1999). Diversity of hydraulic conductance in leaves of temperate and tropical species: possible causes and consequences. J Trop For Sci 11, 47-60. |
| [80] | Uhl D, Mosbrugger V (1999). Leaf venation density as a climate and environmental proxy: a critical review and new data. Palaeogeogr Palaeoclimatol Palaeoecol 149, 15-26. |
| [81] | Van Arendonk JJCM, Poorter H (1994). The chemical composition and anatomical structure of leaves of grass species differing in relative growth rate. Plant Cell Environ 17, 963-970. |
| [82] | Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E (2007). Let the concept of trait be functional! Oikos 116, 882-892. |
| [83] | Westoby M, Reich PB, Wright IJ (2013). Understanding ecological variation across species: area-based vs mass- based expression of leaf traits. New Phytol 199, 322-323. |
| [84] | Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets U, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M (2005). Modulation of leaf economic traits and trait relationships by climate. Glob Ecol Biogeogr 14, 411-421. |
| [85] | Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004). The worldwide leaf economics spectrum. Nature 428, 821-827. |
| [86] | Wright IJ, Westoby M (2002). Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol 155, 403-416. |
| [87] | Xu H, Blonder B, Jodra M, Malhi Y, Fricker M (2021). Automated and accurate segmentation of leaf venation networks via deep learning. New Phytol 229, 631-648. |
| [88] | Zwieniecki MA, Boyce CK, Holbrook NM (2004). Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant Cell Environ 27, 357-365. |
/
| 〈 |
|
〉 |