

基于天然低共熔溶剂的甜叶菊中甜菊糖绿色提取方法及优化
收稿日期: 2021-04-17
录用日期: 2021-09-17
网络出版日期: 2021-09-17
基金资助
上海交通大学农工交叉项目(Agri-X201700X)
Green Extraction Method and Optimization of Steviosides from Stevia rebaudiana by Natural Deep Eutectic Solvent
Received date: 2021-04-17
Accepted date: 2021-09-17
Online published: 2021-09-17
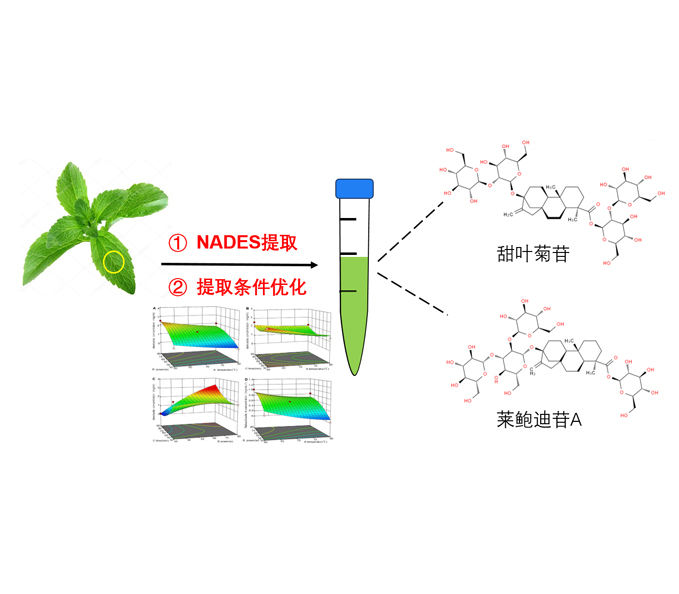
尝试利用天然低共熔溶剂(NADES)提取甜叶菊(Stevia rebaudiana)中的甜菊糖, 探索一种高效、绿色和环保的甜菊糖提取新方法。以甜叶菊干叶为原料, 对照传统提取溶剂水, 以甜菊糖中甜菊苷和莱鲍迪苷A的提取浓度作为指标, 筛选出最优的NADES提取配方, 然后通过Box-Behnken响应面法对NADES提取甜叶菊中甜菊糖的工艺条件进行筛选优化。结果表明, 提取效率最高的NADES配方为1,2-丙二醇:甘油:水=8:1:1 (v/v/v), 提取的甜菊苷浓度为2.59 mg∙mL-1, 比水提取高16.40%, 提取的莱鲍迪苷A浓度为1.06 mg∙mL-1, 比水提取高12.62%; 通过响应面法得到最优提取条件: 提取时间90分钟, 提取温度60°C, 超声功率为80 J∙s-1, 预测甜菊苷提取浓度为3.49 mg∙mL-1, 莱鲍迪苷A提取浓度为1.43 mg∙mL-1, 与实验验证值(甜菊苷浓度为3.48 mg∙mL-1, 莱鲍迪苷A浓度为1.42 mg∙mL-1)接近。在最优条件下, 甜菊苷提取浓度比初始条件提高了34.36%, 莱鲍迪甘A提取浓度比初始条件提高了33.96%。NADES绿色环保, 且提取效率高于传统溶剂, 可用于甜叶菊中甜菊糖的绿色提取; 同时, 该提取方法可为后续推广至其它大宗经济植物类天然产物的绿色工业生产提供参考。
缪晴 , 萨比哈·帕合尔丁 , 曾思瑀 , 潘琪芳 . 基于天然低共熔溶剂的甜叶菊中甜菊糖绿色提取方法及优化[J]. 植物学报, 2021 , 56(6) : 722 -731 . DOI: 10.11983/CBB21064
Natural deep eutectic solvent (NADES) was used to extract steviosides from Stevia rebaudiana as an efficient, green and environmentally friendly new method. Compared with traditional solvent water, the optimal NADES formula was selected to extract S. rebaudiana dry leaves by measuring the concentration of stevioside and rebaudioside A, two main components in steviosides. The extraction conditions of NADES extracting steviosides were optimized by Box-Behnken design from response surface methodology. The best NADES formula for steviosides extraction was selected: 1,2-propanediol:glycerol:water=8:1:1 (v/v/v), the extraction concentration of stevioside was 2.59 mg∙mL-1 (16.40% higher than water), and the extraction concentration of rebaudioside A was 1.06 mg∙mL-1 (12.62% higher than water). The NADES extraction conditions were optimized by response surface methodology: extraction time was 90 min, extraction temperature was 60°C, ultrasonic power was 80 J∙s-1, the predicted extraction concentration of stevioside was 3.49 mg∙mL-1 and the concentration of rebaudioside A was 1.43 mg∙mL-1, which was close to the experimental verification value (the concentration of stevioside is 3.48 mg∙mL-1, the concentration of rebaudioside A is 1.42 mg∙mL-1). Under the best condition, the extraction of stevioside was 34.36% higher than the initial condition, and the extraction of rebaudioside A was 33.96% higher than the initial condition. NADES is environmentally friendly and has higher extraction efficiency than traditional solvents. It can be used for the green extraction of steviosides from S. rebaudiana. At the same time, it provides a promising prospect for the green extraction of natural products from other bulk economic plants.

Key words: NADES; Stevia rebaudiana; stevioside; rebaudioside A; green extraction
| [1] | 陈育如, 杨凤平, 杨帆, 宋婷婷, 束成杰, 尹慧慧 (2016). 甜叶菊及甜菊糖的多效功能与保健应用. 南京师大学报(自然科学版) 39(2), 56-60. |
| [2] | 郎青云, 李慧, 祝谢民, 周艳, 史保国 (2019). 超声辅助纤维素酶提取甜菊糖及其抑菌活性研究. 安徽农学通报 25(21), 30-35. |
| [3] | 刘贵君, 石浩 (2016). 甜菊糖的应用研究进展. 浙江化工 47 (11), 34-41. |
| [4] | 吴则东, 张文彬, 吴玉梅, 刘乃新 (2016). 世界甜叶菊发展概况. 中国糖料 38(4), 62-65. |
| [5] | 严贤春 (2003). 天然甜味剂植物的开发利用研究. 食品研究与开发 24, 59-62. |
| [6] | 应以坚, 王雪奇 (2012). 生物产品甜菊苷综述. 轻工科技 28, 9-10, 53. |
| [7] | 张桂春, 刘玉静, 李延敏, 牟萍, 曲明娟, 李清, 周菊华 (2017). 火龙果果皮中可溶性膳食纤维的提取方法. 植物学报 52, 622-630. |
| [8] | 张杨, 陈天红, 孙君坦, 何炳林 (1998). 甜叶菊糖的组分分离与味质改进研究进展. 化学通报 (6), 11-16. |
| [9] | 赵永良, 韩骁, 刘景彬, 谢印芝 (2010). 膜分离技术在甜菊糖甙提取分离中的应用研究. 化学与生物工程 27, 84-85. |
| [10] | Dai YT, Rozema E, Verpoorte R, Choi YH (2016). Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J Chromatogr A 1434, 50-56. |
| [11] | Dai YT, Witkamp GJ, Verpoorte R, Choi YH (2015). Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem 187, 14-19. |
| [12] | Erkucuk A, Akgun IH, Yesil-Celiktas O (2009). Supercritical CO2 extraction of glycosides from Stevia rebaudiana leaves: identification and optimization. J Supercrit Fluids 51, 29-35. |
| [13] | Gasmalla MAA, Yang RJ, Musa A, Hua X, Ye FY (2017). Influence of sonication process parameters to the state of liquid concentration of extracted rebaudioside A from stevia (Stevia rebaudiana Bertoni) leaves. Arab J Chem 10, 726-731. |
| [14] | Huang Y, Feng F, Jiang J, Qiao Y, Wu T, Voglmeir J, Chen ZG (2017). Green and efficient extraction of rutin from Tartary buckwheat hull by using natural deep eutectic solvents. Food Chem 221, 1400-1405. |
| [15] | Jaitak V, Bandna BS, Kaul VK (2009). An efficient microwave-assisted extraction process of stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni). Phytochem Anal 20, 240-245. |
| [16] | Jentzer JB, Alignan M, Vaca-Garcia C, Rigal L, Vilarem G (2015). Response surface methodology to optimise accelerated solvent extraction of steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem 166, 561-567. |
| [17] | Lemus-Mondaca R, Vega-Gálvez A, Zura-Bravo L, Ah- Hen K (2012). Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects. Food Chem 132, 1121-1132. |
| [18] | Puri M, Sharma D, Barrow CJ, Tiwary AK (2012). Optimisation of novel method for the extraction of steviosides from Stevia rebaudiana leaves. Food Chem 132, 1113-1120. |
/
| 〈 |
|
〉 |