

十字花科植物ABI4序列演化与转录激活活性分析
收稿日期: 2021-02-10
录用日期: 2021-08-09
网络出版日期: 2021-08-11
基金资助
国家自然科学基金(31970228);全国大学生创新创业计划(201910718034)
Analysis on the Evolution and Transcription Activation Activity of ABI4 in Brassicaceae
Received date: 2021-02-10
Accepted date: 2021-08-09
Online published: 2021-08-11
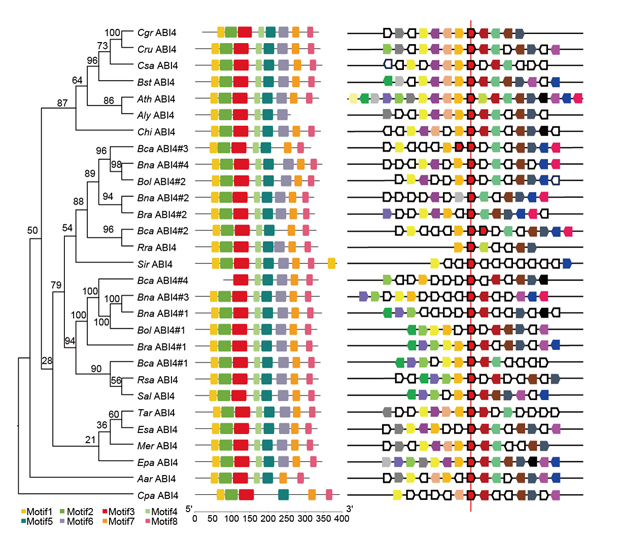
转录因子ABI4参与植物激素ABA的绝大多数生物学功能, 它不仅是ABA和GA在植物体内含量平衡的核心调控因子, 同时还连接ABA与植物细胞内多个信号通路。利用拟南芥(Arabidopsis thaliana) ABI4序列在十字花科其它19种植物(除拟南芥外)中检索得到27个同源基因, 通过序列多态性分析、系统发生重建、染色体共线性分析和转录激活活性比较, 探讨了该基因在十字花科植物中的演化历史。结果表明, ABI4蛋白质序列和结构在十字花科植物中具有较高的保守性, 暗示了其功能的重要性; 单独的ABI4蛋白并不具备显著的转录激活活性, 说明其生物学功能的具体分子机制可能相对复杂, 需要进一步探讨。
张文晶 , 杨晓萌 , 高侃 , 魏欣仪 , 石雪彤 , 王瑞瑄 , 武凤霞 , 康菊清 . 十字花科植物ABI4序列演化与转录激活活性分析[J]. 植物学报, 2021 , 56(6) : 676 -686 . DOI: 10.11983/CBB21036
ABI4 is an important component of the ABA signal transduction pathway. It not only acts as a key player in ABA and GA antagonism, but also plays important roles in various aspects of ABA crosstalk with other signal chemicals. Genes regulated by ABI4 are involved in diverse processes. In this study, we got 27 homologs from 19 species in the family Brassicaceae using AthABI4 as a query, and explored their evolutionary history based on sequence polymorphism analysis, phylogenetic reconstruction, genomic synteny assay, and transcription activation activity comparison. The result revealed that ABI4 are highly conserved and might be not work as transcriptional activators independently in the Brassicaceae, which implies that the irreplaceable roles of ABI4 in plants and further research was needed to explore the molecular mechanism of its biological function.

| [1] | 李媛媛, 傅廷栋, 马朝芝 (2007). 芸薹属植物比较基因组学研究进展. 植物学通报 24, 200-207. |
| [2] | Acevedo-Hernández GJ, León P, Herrera-Estrella LR (2005). Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. Plant J 43, 506-519. |
| [3] | Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leóon P (2000). Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14, 2085-2096. |
| [4] | Bajguz A (2009). Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. J Plant Physiol 166, 882-886. |
| [5] | Bossi F, Cordoba E, Dupré P, Mendoza MS, Román CS, León P (2009). The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59, 359-374. |
| [6] | Finet C, Berne-Dedieu A, Scutt CP, Marlétaz F (2013). Evolution of the ARF gene family in land plants: old domains, new tricks. Mol Biol Evol 30, 45-56. |
| [7] | Finkelstein RR, Gampala SSL, Rock CD (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14, S15-S45. |
| [8] | Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998). The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA2 domain protein. Plant Cell 10, 1043-1054. |
| [9] | Foyer CH, Kerchev PI, Hancock RD (2012). The ABA-INSENSITIVE-4 (ABI4) transcription factor links redox, hormone and sugar signaling pathways. Plant Signal Behav 7, 276-281. |
| [10] | Giraud E, Van Aken O, Ho LHM, Whelan J (2009). The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol 150, 1286-1296. |
| [11] | Hanada K, Hase T, Toyoda T, Shinozaki K, Okamoto M (2011). Origin and evolution of genes related to ABA metabolism and its signaling pathways. J Plant Res 124, 455-465. |
| [12] | Huang CH, Sun RR, Hu Y, Zeng LP, Zhang N, Cai LM, Zhang Q, Koch MA, Al-Shehbaz I, Edger PP, Pires JC, Tan DY, Zhong Y, Ma H (2016). Resolution of Brassicaceae phylogeny using nuclear genes uncovers nested radiations and supports convergent morphological evolution. Mol Biol Evol 33, 394-412. |
| [13] | Ikeda M, Ohme-Takagi M (2009). A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol 50, 970-975. |
| [14] | Kang JQ, Zhang HT, Sun TS, Shi YH, Wang JQ, Zhang BC, Wang ZH, Zhou YH, Gu HY (2013). Natural variation of C-repeat-binding factor (CBFs) genes is a major cause of divergence in freezing tolerance among a group of Arabidopsis thaliana populations along the Yangtze River in China. New Phytol 199, 1069-1080. |
| [15] | Kazan K (2006). Negative regulation of defence and stress genes by EAR-motif-containing repressors. Trends Plant Sci 11, 109-112. |
| [16] | Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH (2011). The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23, 3319-3334. |
| [17] | Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto- Martins G, Surpin M, Lim IJ, Mittler R, Chory J (2007). Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715-719. |
| [18] | Kucera B, Cohn MA, Leubner-Metzger G (2005). Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15, 281-307. |
| [19] | Laby RJ, Kincaid MS, Kim D, Gibson SI (2000). The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23, 587-596. |
| [20] | Liu JY, Osbourn A, Ma PD (2015). MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol Plant 8, 689-708. |
| [21] | Liu SY, Liu YM, Yang XH, Tong CB, Edwards D, Parkin IAP, Zhao MX, Ma JX, Yu JY, Huang SM, Wang XY, Wang JY, Lu K, Fang ZY, Bancroft I, Yang TJ, Hu Q, Wang XF, Yue Z, Li HJ, Yang LF, Wu J, Zhou Q, Wang WX, King GJ, Pires JC, Lu CX, Wu ZY, Sampath P, Wang Z, Guo H, Pan SK, Yang LM, Min JM, Zhang D, Jin DC, Li WS, Belcram H, Tu JX, Guan M, Qi CK, Du DZ, Li JN, Jiang LC, Batley J, Sharpe AG, Park BS, Ruperao P, Cheng F, Waminal NE, Huang Y, Dong CH, Wang L, Li JP, Hu ZY, Zhuang M, Huang Y, Huang JY, Shi JQ, Mei DS, Liu J, Lee TH, Wang JP, Jin HZ, Li ZY, Li X, Zhang JF, Xiao L, Zhou YM, Liu ZS, Liu XQ, Qin R, Tang X, Liu WB, Wang YP, Zhang YY, Lee J, Kim HH, Denoeud F, Xu X, Liang XM, Hua W, Wang XW, Wang J, Chalhoub B, Paterson AH (2014). The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5, 3930. |
| [22] | Lysak MA, Koch MA, Pecinka A, Schubert I (2005). Chromosome triplication found across the tribe Brassiceae. Geno Res 15, 516-525. |
| [23] | Ma DW, Constabel CP (2019). MYB repressors as regulators of phenylpropanoid metabolism in plants. Trends Plant Sci 24, 275-289. |
| [24] | Matsui K, Umemura Y, Ohme-Takagi M (2008). AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J 55, 954-967. |
| [25] | Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012). AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta (BBA)-Gene Regul Mech 1819, 86-96. |
| [26] | Nakamura S, Lynch TJ, Finkelstein RR (2001). Physical interactions between ABA response loci of Arabidopsis. Plant J 26, 627-635. |
| [27] | Nakano T, Suzuki K, Fujimura T, Shinshi H (2006). Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140, 411-432. |
| [28] | Niu XP, Helentjaris T, Bate NJ (2002). Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell 14, 2565-2575. |
| [29] | Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M (2001). Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13, 1959-1968. |
| [30] | Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, Palme K (2009). The evolution of nuclear auxin signaling. BMC Evol Biol 9, 126. |
| [31] | Reeves WM, Lynch TJ, Mobin R, Finkelstein RR (2011). Direct targets of the transcription factors ABA-insensitive (ABI) 4 and ABI5 reveal synergistic action by ABI4 and several bZIP ABA response factors. Plant Mol Biol 75, 347-363. |
| [32] | Shkolnik-Inbar D, Bar-Zvi D (2010). ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22, 3560-3573. |
| [33] | Shu K, Chen Q, Wu YR, Liu RJ, Zhang HW, Wang PF, Li YL, Wang SF, Tang SY, Liu CY, Yang WY, Cao XF, Serino G, Xie Q (2016a). ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J 85, 348-361. |
| [34] | Shu K, Liu XD, Xie Q, He ZH (2016b). Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant 9, 34-45. |
| [35] | Shu K, Zhang HW, Wang SF, Chen ML, Wu YR, Tang SY, Liu CY, Feng YQ, Cao XF, Xie Q (2013). ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet 9, e1003577. |
| [36] | Shu K, Zhou WG, Chen F, Luo XF, Yang WY (2018a). Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front Plant Sci 9, 416. |
| [37] | Shu K, Zhou WG, Yang WY (2018b). APETALA 2-domain- containing transcription factors: focusing on abscisic acid and gibberellins antagonism. New Phytol 217, 977-983. |
| [38] | So?derman EM, Brocard IM, Lynch TJ, Finkelstein RR (2000). Regulation and function of the Arabidopsis ABA- insensitive 4 gene in seed and abscisic acid response signaling networks. Plant Physiol 124, 1752-1765. |
| [39] | Sun XW, Feng PQ, Xu XM, Guo HL, Ma JF, Chi W, Lin RC, Lu CM, Zhang LX (2011). A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat Commun 2, 477. |
| [40] | Talboys PJ, Zhang HM, Knox JP (2011). ABA signaling modulates the detection of the LM6 arabinan cell wall epitope at the surface of Arabidopsis thaliana seedling root apices. New Phytol 190, 618-626. |
| [41] | Ton J, Mauch-Mani B (2004). β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38, 119-130. |
| [42] | Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010). Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51, 1821-1839. |
| [43] | Wang XW, Wang HZ, Wang J, Sun RF, Wu J, Liu SY, Bai YQ, Mun JH, Bancroft I, Cheng F, Huang SW, Li XX, Hua W, Wang JY, Wang XY, Freeling M, Pires JC, Paterson AH, Chalhoub B, Wang B, Hayward A, Sharpe AG, Park BS, Weisshaar B, Liu BH, Li B, Liu B, Tong CB, Song C, Duran C, Peng CF, Geng CY, Koh C, Lin CY, Edwards D, Mu DS, Shen D, Soumpourou E, Li F, Fraser F, Conant G, Lassalle G, King GJ, Bonnema G, Tang HB, Wang HP, Belcram H, Zhou HL, Hirakawa H, Abe H, Guo H, Wang H, Jin HZ, Parkin IAP, Batley J, Kim JS, Just J, Li JW, Xu JH, Deng J, Kim JA, Li JP, Yu JY, Meng JL, Wang JP, Min JM, Poulain J, Wang J, Hatakeyama K, Wu K, Wang L, Fang L, Trick M, Links MG, Zhao MX, Jin MN, Ramchiary N, Drou N, Berkman PJ, Cai QL, Huang QF, Li RQ, Tabata S, Cheng SF, Zhang S, Zhang SJ, Huang SM, Sato S, Sun SL, Kwon SJ, Choi SR, Lee TH, Fan W, Zhao X, Tan X, Xu X, Wang Y, Qiu Y, Yin Y, Li YR, Du YC, Liao YC, Lim Y, Narusaka Y, Wang YP, Wang ZY, Li ZY, Wang ZW, Xiong ZY, Zhang ZH (2011). The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43, 1035-1039. |
| [44] | Wind JJ, Peviani A, Snel B, Hanson J, Smeekens SC (2013). ABI4: versatile activator and repressor. Trends Plant Sci 18, 125-132. |
| [45] | Yaish MW, El-kereamy A, Zhu T, Beatty PH, Good AG, Bi YM, Rothstein SJ (2010). The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet 6, e1001098. |
| [46] | Yang Y, Yu XC, Song LF, An CC (2011). ABI4 activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol 156, 873-883. |
| [47] | Ye R, Yao QH, Xu ZH, Xue HW (2004). Development of an efficient method for the isolation of factors involved in gene transcription during rice embryo development. Plant J 38, 348-357. |
/
| 〈 |
|
〉 |