

P515的测量原理、方法和应用
收稿日期: 2021-03-23
录用日期: 2021-06-18
网络出版日期: 2021-06-18
基金资助
中国科学院仪器设备功能开发技术创新项目(2018g0048)
The Measurement Principles, Methods and Applications of P515
Received date: 2021-03-23
Accepted date: 2021-06-18
Online published: 2021-06-18
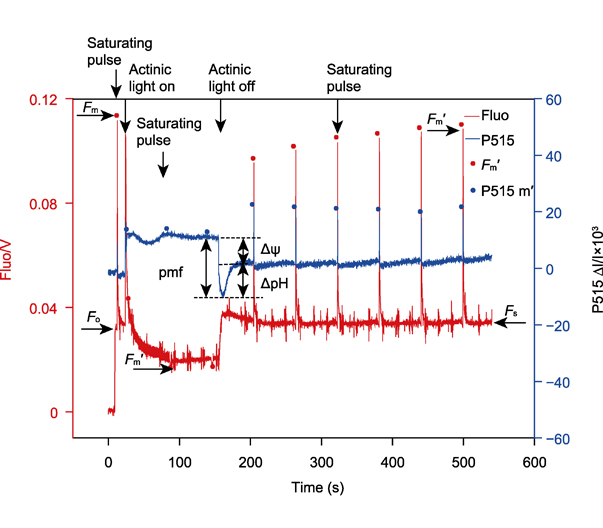
光谱技术已广泛应用于光合研究领域, 如光吸收信号P515和P700氧化还原动力学以及叶绿素荧光等, 可快速、准确地检测植物的光合活性。P515信号广泛存在于高等植物和藻类中, 是类囊体膜上的色素分子吸收光能后, 其吸收光谱发生位移造成。利用光诱导的P515快速和慢速动力学, 可检测PSI和PSII反应中心的比值、ATP合酶的质子传导性、围绕PSI的环式电子传递速率、质子动力势及其组分, 还可通过同步检测叶绿素荧光和P515信号研究光保护机制。该文总结了P515的主要测量原理、方法及其应用, 旨在为深入研究光合作用机理提供技术支持。
张春艳 , 庞肖杰 . P515的测量原理、方法和应用[J]. 植物学报, 2021 , 56(5) : 594 -604 . DOI: 10.11983/CBB21052
The spectral techniques have been widely used in the field of photosynthesis research, such as the light absorption signals P515 and P700 redox kinetics, and chlorophyll fluorescence, which can detect the photosynthetic activities of plants quickly and accurately. P515 signal is widely present in higher plants and algae, which is caused by the shift of absorption spectrum of pigments on thylakoid membrane. We can detect the ratio of PSI to PSII reaction center, the proton conductivity of chloroplast ATP synthase, the cyclic electron flow rate around PSI, the proton motive force (pmf) and its components by the P515 fast and slow kinetics, and study the photoprotective mechanism by simultaneous detection of P515 signal and chlorophyll fluorescence. In this paper, we summarize the main measurement methods of P515, expound its principles, and the applications. The aim is to provide technical supports for further study on the mechanism of photosynthesis.

Key words: P515; ATP synthase; cyclic electron transport; proton motive force
| [1] | 付振书, 赵世杰, 孟庆伟 (2004). 类囊体腔的酸化与过剩激发能耗散. 植物学通报 21, 486-494. |
| [2] | Allorent G, Byrdin M, Carraretto L, Morosinotto T, Szabo I, Finazzi G (2018). Global spectroscopic analysis to study the regulation of the photosynthetic proton motive force: a critical reappraisal. Biochim Biophys Acta Bioenerg 1859, 676-683. |
| [3] | Alric J (2014). Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii: (II) involvement of the PGR5-PGRL1 pathway under anaerobic conditions. Biochim Biophys Acta Bioenerg 1837, 825-834. |
| [4] | Bailleul B, Cardol P, Breyton C, Finazzi G (2010). Electrochromism: a useful probe to study algal photosynthesis. Photosynth Res 106, 179-189. |
| [5] | Baker NR, Harbinson J, Kramer DM (2007). Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30, 1107-1125. |
| [6] | Bennoun P (1994). Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas. Biochim Biophys Acta Bioenerg 1186, 59-66. |
| [7] | Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M (2012). Acclimation of Chlamydomonas reinhardtii to different growth irradiances. J Biol Chem 287, 5833-5847. |
| [8] | Bujaldon S, Kodama N, Rathod MK, Tourasse N, Ozawa SI, Sellés J, Vallon O, Takahashi Y, Wollman FA (2020). The BF4 and p71 antenna mutants from Chlamydomonas reinhardtii. Biochim Biophys Acta Bioenerg 1861, 148085. |
| [9] | Carraretto L, Formentin E, Teardo E, Checchetto V, Tomizioli M, Morosinotto T, Giacometti GM, Finazzi G, Szabó I (2013). A thylakoid-located two-pore K+ channel controls photosynthetic light utilization in plants. Science 342, 114-118. |
| [10] | Carraretto L, Teardo E, Checchetto V, Finazzi G, Uozumi N, Szabo I (2016). Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: from molecular identification to function. Mol Plant 9, 371-395. |
| [11] | Checchetto V, Teardo E, Carraretto L, Formentin E, Bergantino E, Giacometti GM, Szabo I (2013). Regulation of photosynthesis by ion channels in cyanobacteria and higher plants. Biophys Chem 182, 51-57. |
| [12] | Checchetto V, Teardo E, Carraretto L, Leanza L, Szabo I (2016). Physiology of intracellular potassium channels: a unifying role as mediators of counterion fluxes? Biochim Biophys Acta Bioenerg 1857, 1258-1266. |
| [13] | Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001). Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry 40, 1226-1237. |
| [14] | Davis GA, Kanazawa A, Schöttler MA, Kohzuma K, Froehlich JE, Rutherford AW, Satoh-Cruz M, Minhas D, Tietz S, Dhingra A, Kramer DM (2016). Limitations to photosynthesis by proton motive force-induced photosystem II photodamage. eLife 5, e16921. |
| [15] | Davis GA, Rutherford AW, Kramer DM (2017). Hacking the thylakoid proton motive force for improved photosynthesis: modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Philos Trans R Soc Lond B Biol Sci 372, 20160381. |
| [16] | de Bianchi S, Ballottari M, Dall'Osto L, Bassi R (2010). Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans 38, 651-660. |
| [17] | Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012). Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113, 75-88. |
| [18] | Duan ZK, Kong FN, Zhang L, Li WJ, Zhang J, Peng LW (2016). A bestrophin-like protein modulates the proton motive force across the thylakoid membrane in Arabidopsis. J Integr Plant Biol 58, 848-858. |
| [19] | Duysens LNM (1954). Reversible changes in the absorption spectrum of Chlorella upon irradiation. Science 120, 353-354. |
| [20] | Fork DC, Amesz J (1967). Light-induced shifts in the absorption spectrum of carotenoids in red and brown algae. Photochem Photobiol 6, 913-918. |
| [21] | Frese RN, Palacios MA, Azzizi A, van Stokkum IHM, Kruip J, Rögner M, Karapetyan NV, Schlodder E, van Grondelle R, Dekker JP (2002). Electric field effects on red chlorophylls, β-carotenes and P700 in cyanobacterial Photosystem I complexes. Biochim Biophys Acta Bioenerg 1554, 180-191. |
| [22] | Fristedt R, Martins NF, Strenkert D, Clarke CA, Suchoszek M, Thiele W, Schöttler MA, Merchant SS (2015). The thylakoid membrane protein CGL160 supports CF1CFo ATP synthase accumulation in Arabidopsis thaliana. PLoS One 10, e0121658. |
| [23] | Hind G, Nakatani HY, Izawa S (1974). Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci USA 71, 1484-1488. |
| [24] | Hirano M, Katoh S (1981). Electrochromic band shifts of carotenoid in a blue-green alga. Photochem Photobiol 34, 637-643. |
| [25] | Holmes NG, Hunter CN, Niederman RA, Crofts AR (1980). Identification of the pigment pool responsible for the flash-induced carotenoid band shift in Rhodopseudomonas sphaeroides chromatophores. FEBS Lett 115, 43-48. |
| [26] | Joliot P, Joliot A (2002). Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99, 10209-10214. |
| [27] | Junge W, Witt HT (1968). On the ion transport system of photosynthesis: investigations on a molecular level. Z Naturforsch B 23, 244-254. |
| [28] | Klughammer C, Siebke K, Schreiber U (2013). Continuous ECS-indicated recording of the proton-motive charge flux in leaves. Photosynth Res 117, 471-487. |
| [29] | Kramer DM, Avenson TJ, Edwards GE (2004). Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9, 349-357. |
| [30] | Kramer DM, Cruz JA, Kanazawa A (2003). Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 8, 27-32. |
| [31] | Kramer DM, Evans JR (2011). The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155, 70-78. |
| [32] | Kramer H, Mathis P (1980). Quantum yield and rate of formation of the carotenoid triplet state in photosynthetic structures. Biochim Biophys Acta Bioenerg 593, 319-329. |
| [33] | Liguori N, Roy LM, Opacic M, Durand G, Croce R (2013). Regulation of light harvesting in the green alga Chlamydomonas reinhardtii: the C-terminus of LHCSR is the knob of a dimmer switch. J Am Chem Soc 135, 18339-18342. |
| [34] | Lucker B, Kramer DM (2013). Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth Res 117, 449-459. |
| [35] | Nandha B, Finazzi G, Joliot P, Hald S, Johnson GN (2007). The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim Biophys Acta Bioenerg 1767, 1252-1259. |
| [36] | Nawrocki WJ, Santabarbara S, Mosebach L, Wollman FA, Rappaport F (2016). State transitions redistribute rather than dissipate energy between the two photosystems in Chlamydomonas. Nat Plants 2, 16031. |
| [37] | Niyogi KK, Grossman AR, Björkman O (1998). Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121-1134. |
| [38] | Pottosin II, Schönknecht G (1996). Ion channel permeable for divalent and monovalent cations in native spinach thylakoid membranes. J Membr Biol 152, 223-233. |
| [39] | Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schöttler MA (2011). ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23, 304-321. |
| [40] | Ruban AV, Pascal AA, Robert B, Horton P (2002). Activation of zeaxanthin is an obligatory event in the regulation of photosynthetic light harvesting. J Biol Chem 277, 7785-7789. |
| [41] | Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM (2000). The proton to electron stoichiometry of steady- state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. Proc Natl Acad Sci USA 97, 14283-14288. |
| [42] | Sacksteder CA, Kramer DM (2000). Dark-interval relaxation kinetics (DIRK) of absorbance changes as a quantitative probe of steady-state electron transfer. Photosynth Res 66, 145-158. |
| [43] | Schmidt S, Reich R, Witt HT (1971). Electrochromism of chlorophylls and carotenoids in multilayers and in chloroplasts. Naturwissenschaften 58, 414. |
| [44] | Schönknecht G, Hedrich R, Junge W, Raschke K (1988). A voltage-dependent chloride channel in the photosynthetic membrane of a higher plant. Nature 336, 589-592. |
| [45] | Schreiber U, Klughammer C (2008). New accessory for the DUAL-PAM-100: the P515/535 module and examples of its application. PAM Appl Notes 1, 1-10. |
| [46] | Sonoike K (2011). Photoinhibition of photosystem I. Physiol Plant 142, 56-64. |
| [47] | Spetea C, Herdean A, Allorent G, Carraretto L, Finazzi G, Szabo I (2017). An update on the regulation of photosynthesis by thylakoid ion channels and transporters in Arabidopsis. Physiol Plant 161, 16-27. |
| [48] | Sukhov V, Surova L, Morozova E, Sherstneva O, Vodeneev V (2016). Changes in H+-ATP synthase activity, proton electrochemical gradient, and pH inPea chloroplast can be connected with variation potential. Front Plant Sci 7, 1092. |
| [49] | Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013). Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4, 1954. |
| [50] | Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007). The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta Bioenerg 1767, 1233-1244. |
| [51] | Tolleter D, Ghysels B, Alric J, Petroutsos D, Tolstygina I, Krawietz D, Happe T, Auroy P, Adriano JM, Beyly A, Cuiné S, Plet J, Reiter IM, Genty B, Cournac L, Hippler M, Peltier G (2011). Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23, 2619-2630. |
| [52] | Viola S, Bailleul B, Yu JF, Nixon P, Sellés J, Joliot P, Wollman FA (2019). Probing the electric field across thylakoid membranes in cyanobacteria. Proc Natl Acad Sci USA 116, 21900-21906. |
| [53] | Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi HL (2006). Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol 141, 465-474. |
| [54] | Witt HT (1979). Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods: the central role of the electric field. Biochim Biophys Acta 505, 355-427. |
| [55] | Yamori W, Takahashi S, Makino A, Price GD, Badger MR, von Caemmerer S (2011). The roles of ATP synthase and the cytochrome b 6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol 155, 956-962. |
| [56] | Zhang L, Duan ZK, Zhang J, Peng LW (2016). Biogenesis factor required for ATP synthase 3 facilitates assembly of the chloroplast ATP synthase complex. Plant Physiol 171, 1291-1306. |
| [57] | Zhang L, Pu H, Duan ZK, Li YH, Liu B, Zhang QQ, Li WJ, Rochaix JD, Liu L, Peng LW (2018). Nucleus-encoded protein BFA1 promotes efficient assembly of the chloroplast ATP synthase coupling factor 1. Plant Cell 30, 1770-1788. |
| [58] | Zhang R, Cruz JA, Kramer DM, Magallanes-Lundback ME, Dellapenna D, Sharkey TD (2009). Moderate heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted, intact tobacco leaves. Plant Cell Environ 32, 1538-1547. |
| [59] | Zoschke R, Kroeger T, Belcher S, Schöttler MA, Barkan A, Schmitz-Linneweber C (2012). The pentatricopeptide repeat-SMR protein ATP4 promotes translation of the chloroplast atpB/E mRNA. Plant J 72, 547-558. |
/
| 〈 |
|
〉 |