

模式识别受体的胞内转运及其在植物免疫中的作用
收稿日期: 2019-07-28
录用日期: 2020-02-26
网络出版日期: 2020-02-26
基金资助
国家自然科学基金(31622005);北京林业大学优秀青年人才培育项目(2019JQ03003)
Intracellular Trafficking in Pattern Recognition Receptor-triggered Plant Immunity
Received date: 2019-07-28
Accepted date: 2020-02-26
Online published: 2020-02-26
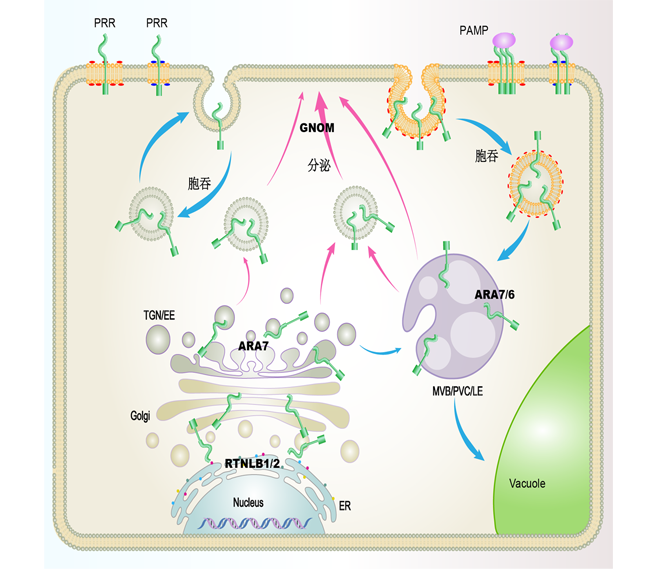
植物利用细胞表面模式识别受体(PRRs)来感知病原相关分子模式(PAMPs), 进而触发自身的免疫反应(PTI)。在植物免疫过程中, PRRs在细胞内的正确定位对其生理功能的发挥至关重要。PRRs蛋白可以在内质网(ER)上合成, 并通过胞吐被分泌到质膜(PM)上。此外, PRRs蛋白也可以通过胞吞进行胞内循环或降解。细胞可以通过胞内转运降解PRRs蛋白以终止信号转导, 也可以通过形成胞内体进行信号传递。该文概述了PRRs蛋白及其配体的研究进展以及PRRs蛋白的胞内转运在植物免疫中的重要作用。
崔亚宁 , 钱虹萍 , 赵艳霞 , 李晓娟 . 模式识别受体的胞内转运及其在植物免疫中的作用[J]. 植物学报, 2020 , 55(3) : 329 -339 . DOI: 10.11983/CBB19139
Plants initially sense microbes via perception of pathogen associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) located on the cell surface. This recognition is referred to as PAMP-triggered immunity (PTI). In order to ensure their physiological and cellular functions, PRRs must be properly conveyed from their site of synthesis, i.e., the endoplasmic reticulum, to their final destination, the plasma membrane (PM), through the secretory pathway. PRRs also rely on recycling and/or degradation, two processes that are initiated by endocytosis. Intracellular trafficking serves to terminate signaling through degradation, sustains signaling through recycling, or relays signaling inside the cell through the formation of signaling endosomes. In this review, we summarize the current knowledge of plant PRRs and their ligands, illustrating that intracellular trafficking plays an important role in plant immunity.

| [1] | 季东超, 宋凯, 邢晶晶, 陈彤, 田世平 (2015). LysM蛋白介导植物免疫防卫反应及其信号激发的研究进展. 植物学报 50, 628-636. |
| [2] | Abas L, Benjamins R, Malenica N, Paciorek T, Wi?niewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8, 249-256. |
| [3] | Akamatsu A, Wong HL, Fujiwara M, Okuda J, Nishide K, Uno K, Imai K, Umemura K, Kawasaki T, Kawano Y, Shimamoto K (2013). An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe 13, 465-476. |
| [4] | Barberon M, Zelazny E, Robert S, Conejero G, Curie C, Friml J, Vert G (2011). Monoubiquitin-dependent endocytosis of the IRON-REGULATED TRANSPORTER 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA 108, E450-E458. |
| [5] | Beck M, Heard W, Mbengue M, Robatzek S (2012a). The INs and OUTs of pattern recognition receptors at the cell surface. Curr Opin Plant Biol 15, 367-374. |
| [6] | Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S (2012b). Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell 24, 4205-4219. |
| [7] | Ben Khaled S, Postma J, Robatzek S (2015). A moving view: subcellular trafficking processes in pattern recognition receptor-triggered plant immunity. Annu Rev Phytopathol 53, 379-402. |
| [8] | Bi GZ, Zhou ZY, Wang WB, Li L, Rao SF, Wu Y, Zhang XJ, Menke FLH, Chen S, Zhou JM (2018). Receptor-like cytoplasmic kinases directly link diverse pattern recognition receptors to the activation of mitogen-activated protein kinase cascades in Arabidopsis. Plant Cell 30, 1543-1561. |
| [9] | Boller T, Felix G (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60, 379-406. |
| [10] | Boudsocq M, Willmann MR, McCormack M, Lee H, Shan LB, He P, Bush J, Cheng SH, Sheen J (2010). Differential innate immune signaling via Ca2+ sensor protein kinases . Nature 464, 418-422. |
| [11] | Boutrot F, Zipfel C (2017). Function, discovery, and exploitation of plant pattern recognition receptors for broad- spectrum disease resistance. Annu Rev Phytopathol 55, 257-286. |
| [12] | Bozsoki Z, Cheng J, Feng F, Gysel K, Vinther M, Andersen KR, Oldroyd G, Blaise M, Radutoiu S, Stougaard J (2017). Receptor-mediated chitin perception in legume roots is functionally separable from Nod factor perception. Proc Natl Acad Sci USA 114, E8118-E8127. |
| [13] | Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C (2017). Plant immune and growth receptors share common signaling components but localise to distinct plasma membrane nanodomains. eLife 6, e25114. |
| [14] | Carrasco SE, Yang YY, Troxell B, Yang XL, Pal U, Yang XF (2015). Borrelia burgdorferi elongation factor EF-Tu is an immunogenic protein during Lyme borreliosis. Emerg Microbes Infec 4, 1-8. |
| [15] | Chen LT, Hamada S, Fujiwara M, Zhu TH, Thao N, Wong HL, Krishna P, Ueda T, Kaku H, Shibuya N, Kawasaki T, Shimamoto K (2010). The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe 7, 185-196. |
| [16] | Choi SW, Tamaki T, Ebine K, Uemura T, Ueda T, Nakano A (2013). RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING 2 receptor. Plant Cell 25, 1174-1187. |
| [17] | Couto D, Zipfel C (2016). Regulation of pattern recognition receptor signaling in plants. Nat Rev Immunol 16, 537-552. |
| [18] | Cui YN, Li XJ, Yu M, Li RL, Fan LS, Zhu YF, Lin JX (2018). Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis. Development 145, dev165688. |
| [19] | Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt JA (2009). Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc Natl Acad Sci USA 106, 16428-16433. |
| [20] | Epel BL (1994). Plasmodesmata: composition, structure and trafficking. Plant Mol Biol 26, 1343-1356. |
| [21] | Faulkner C, Petutschnig E, Benitez-Alfonso Y, Beck M, Robatzek S, Lipka V, Maule AJ (2013). LYM2-dependent chitin perception limits molecular flux via plasmodesmata. Proc Natl Acad Sci USA 110, 9166-9170. |
| [22] | Furman-Matarasso N, Cohen E, Du QS, Chejanovsky N, Hanania U, Avni A (1999). A point mutation in the ethylene-inducing xylanase elicitor inhibits the beta-1,4-endoxylanase activity but not the elicitation activity. Plant Physiol 121, 345-352. |
| [23] | Guigón-López C, Guerrero-Prieto V, Lanzuise S, Lorito M (2014). Enzyme activity of extracellular protein induced in Trichoderma asperellum and T. longibrachiatum by substrates based on Agaricus bisporus and Phymatotrichopsis omnivora. Fungal Biol 118, 211-221. |
| [24] | Hann DR, Rathjen JP (2007). Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J 49, 607-618. |
| [25] | Hayafune M, Berisio R, Marchetti R, Silipo A, Kayama M, Desaki Y, Arima S, Squeglia F, Ruggiero A, Tokuyasu K, Molinaro A, Kaku H, Shibuya N (2014). Chitin-induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc Natl Acad Sci USA 111, E404-E413. |
| [26] | Henty-Ridilla JL, Shimono M, Li JJ, Chang JH, Day B, Staiger CJ (2013). The plant actin cytoskeleton responds to signals from microbe-associated molecular patterns. PLoS Pathog 9, e1003290. |
| [27] | Herberth S, Shahriari M, Bruderek M, Hessner F, Müller B, Hülskamp M, Schellmann S (2012). Artificial ubiquitylation is sufficient for sorting of a plasma membrane ATPase to the vacuolar lumen of Arabidopsis cells. Planta 236, 63-77. |
| [28] | Hohmann U, Lau K, Hothorn M (2017). The structural basis of ligand perception and signal activation by receptor kinases. Annu Rev Plant Biol 68, 109-137. |
| [29] | Huffaker A, Pearce G, Ryan CA (2006). An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA 103, 10098-10103. |
| [30] | Jarsch IK, Konrad SSA, Stratil TF, Urbanus SL, Szymanski W, Braun P, Braun KH, Ott T (2014). Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana. Plant Cell 26, 1698-1711. |
| [31] | Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N (2006). Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor . Proc Natl Acad Sci USA 103, 11086-11091. |
| [32] | Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T (2011). High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem 286, 6175-6183. |
| [33] | Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Grossniklaus U, Schulze WX, Robatzek S, Panstruga R (2010). PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem 285, 39140-39149. |
| [34] | Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G (2004). The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16, 3496-3507. |
| [35] | Lee HY, Bowen CH, Popescu GV, Kang HG, Kato N, Ma SS, Dinesh-Kumar S, Snyder M, Popescu SC (2011). Arabidopsis RTNLB1 and RTNLB2 reticulon-like proteins regulate intracellular trafficking and activity of the FLS2 immune receptor. Plant Cell 23, 3374-3391. |
| [36] | Leibman-Markus M, Schuster S, Avni A (2017). LeEIX2 interactors’ analysis and EIX-mediated responses measurement. In: Shan LB, He P, eds. Plant Pattern Recognition Receptors: Methods and Protocols. New York: Humana Press. pp. 167-172. |
| [37] | Liang XX, Ding PT, Lian KH, Wang JL, Ma MM, Li L, Li L, Li M, Zhang XJ, Chen S, Zhang YL, Zhou JM (2016). Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. eLife 5, e13568. |
| [38] | Liang XX, Ma MM, Zhou ZY, Wang JH, Yang XR, Rao SF, Bi GZ, Li L, Zhang XJ, Chai JJ, Chen S, Zhou JM (2018). Ligand-triggered de-repression of Arabidopsis heterotrimeric G proteins coupled to immune receptor kinases. Cell Res 28, 529-543. |
| [39] | Liang XX, Zhou JM (2018). Receptor-like cytoplasmic kinases: central players in plant receptor kinase-mediated signaling. Annu Rev Plant Biol 69, 267-299. |
| [40] | Liu TT, Liu ZX, Song CJ, Hu YF, Han ZF, She J, Fan FF, Wang JW, Jin CW, Chang JB, Zhou JM, Chai JJ (2012). Chitin-induced dimerization activates a plant immune receptor. Science 336, 1160-1164. |
| [41] | Lori M, van Verk MC, Hander T, Schatowitz H, Klauser D, Flury P, Gehring CA, Boller T, Bartels S (2015). Evolutionary divergence of the plant elicitor peptides (Peps) and their receptors: interfamily incompatibility of perception but compatibility of downstream signaling. J Exp Bot 66, 5315-5325. |
| [42] | Lu DP, Lin WW, Gao XQ, Wu SJ, Cheng C, Avila J, Heese A, Devarenne TP, He P, Shan LB (2011). Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science 332, 1439-1442. |
| [43] | Mbengue M, Bourdais G, Gervasi F, Beck M, Zhou J, Spallek T, Bartels S, Boller T, Ueda T, Kuhn H, Robatzek S (2016). Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc Natl Acad Sci USA 113, 11034-11039. |
| [44] | Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006). Plant stomata function in innate immunity against bacterial invasion. Cell 126, 969-980. |
| [45] | Mithoe SC, Ludwig C, Pel MJC, Cucinotta M, Casartelli A, Mbengue M, Sklenar J, Derbyshire P, Robatzek S, Pieterse CMJ, Aebersold R, Menke FLH (2016). Attenua-tion of pattern recognition receptor signaling is mediated by a MAP kinase kinase kinase. EMBO Rep 17, 441-454. |
| [46] | Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104, 19613-19618. |
| [47] | Ortiz-Morea FA, Savatin DV, Dejonghe W, Kumar R, Luo Y, Adamowski M, Van den Begin J, Dressano K, de Oliveira GP, Zhao XY, Lu Q, Madder A, Friml J, de Moura DS, Russinova E (2016). Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc Natl Acad Sci USA 113, 11028-11033. |
| [48] | Robatzek S, Chinchilla D, Boller T (2006). Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20, 537-542. |
| [49] | Schoonbeek HJ, Wang HH, Stefanato FL, Craze M, Bowden S, Wallington E, Zipfel C, Ridout CJ (2015). Arabidopsis EF-Tu receptor enhances bacterial disease resistance in transgenic wheat. New Phytol 206, 606-613. |
| [50] | Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D (2010). Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285, 9444-9451. |
| [51] | Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C (2011). Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7, e1002046. |
| [52] | Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP (2011). Hierarchy and roles of pathogen- associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol 156, 687-699. |
| [53] | Sharfman M, Bar M, Ehrlich M, Schuster S, Melech-Bonfil S, Ezer R, Sessa G, Avni A (2011). Endosomal signaling of the tomato leucine-rich repeat receptor-like protein LeEix2. Plant J 68, 413-423. |
| [54] | Sitia R, Braakman I (2003). Quality control in the endoplasmic reticulum protein factory. Nature 426, 891-894. |
| [55] | Smith JM, Leslie ME, Robinson SJ, Korasick DA, Zhang T, Backues SK, Cornish PV, Koo AJ, Bednarek SY, Heese A (2014). Loss of Arabidopsis thaliana dynamin-related protein 2B reveals separation of innate immune signaling pathways. PLoS Pathog 10, e1004578. |
| [56] | Sparkes I, Tolley N, Aller I, Svozil J, Osterrieder A, Botchway S, Mueller C, Frigerio L, Hawes C (2010). Five Arabidopsis reticulon isoforms share endoplasmic reticulum location, topology, and membrane-shaping properties. Plant Cell 22, 1333-1343. |
| [57] | Spitzer C, Reyes FC, Buono R, Sliwinski MK, Haas TJ, Otegui MS (2009). The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 21, 749-766. |
| [58] | Vitale A, Boston RS (2008). Endoplasmic reticulum quality control and the unfolded protein response: insights from plants. Traffic 9, 1581-1588. |
| [59] | Wang JL, Grubb LE, Wang JY, Liang XX, Li L, Gao CL, Ma MM, Feng F, Li M, Li L, Zhang XJ, Yu FF, Xie Q, Chen S, Zipfel C, Monaghan J, Zhou JM (2018). A regulatory module controlling homeostasis of a plant immune kinase. Mol Cell 69, 493-504. |
| [60] | Wyrsch I, Domínguez-Ferreras A, Geldner N, Boller T (2015). Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytol 206, 774-784. |
| [61] | Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA (2010). PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 22, 508-522. |
| [62] | Yeh YH, Chang YH, Huang PY, Huang JB, Zimmerli L (2015). Enhanced Arabidopsis pattern-triggered immunity by overexpression of cysteine-rich receptor-like kinases. Front Plant Sci 6, 322. |
| [63] | Yeh YH, Panzeri D, Kadota Y, Huang YC, Huang PY, Tao CN, Roux M, Chien HC, Chin TC, Chu PW, Zipfel C, Zimmerli L (2016). The Arabidopsis malectin-like/LRR- RLK IOS1 is critical for BAK1-dependent and BAK1-independent pattern-triggered immunity. Plant Cell 28, 1701-1721. |
| [64] | Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125, 749-760. |
| [65] | Zipfel C, Oldroyd GED (2017). Plant signaling in symbiosis and immunity. Nature 543, 328-336. |
| [66] | Zipfel C, Robatzek S (2010). Pathogen-associated molecular pattern-triggered immunity: veni, vidi..? Plant Physiol 154, 551-554. |
/
| 〈 |
|
〉 |