

蔗糖对桃幼苗生长发育及其SnRK1酶活性的影响
收稿日期: 2019-02-22
录用日期: 2019-05-17
网络出版日期: 2019-05-23
基金资助
国家现代农业产业技术体系建设专项资金(No.CARS-30-2-02);山东省自然科学基金(No.ZR2017BC017);山东省“双一流”建设奖补资金(No.SYL2017YSTD10)
Effects of Sucrose on Seedling Growth and Development and SnRK1 Activity in Prunus persica
Received date: 2019-02-22
Accepted date: 2019-05-17
Online published: 2019-05-23
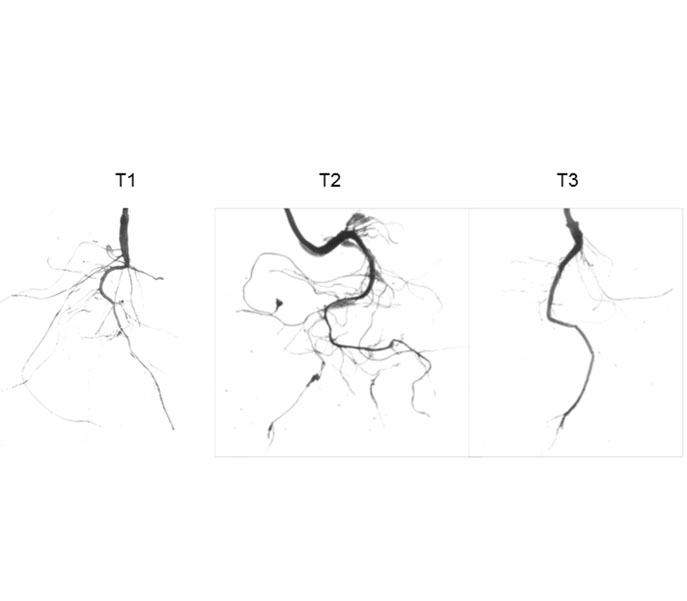
以实生桃(Prunus persica)苗为试材, 探讨SnRK1对不同浓度蔗糖及处理时间的响应特性, 揭示蔗糖对植株生长发育的影响, 以期为果树生产提供理论依据及技术支持。结果表明, 施加5%蔗糖时, 植株体内SnRK1酶活性最高, 且在一定时间内, 酶活性持续升高; 与对照(清水和甘露醇)相比, 5%蔗糖处理显著提高植株可溶性糖、淀粉和叶片叶绿素含量, 增加植株地上部和地下部生物量, 显著加快植株净光合速率; 通过观察根系构型, 发现5%蔗糖可以显著增加根系总表面积、总体积和侧根数量, 并可促进根系加粗加长生长。qRT-PCR分析表明, 外源蔗糖能促进根系中生长素的合成和转运。综上, 一定浓度蔗糖可以提高植株体内SnRK1酶活性, 影响植株碳代谢, 促进植株生长发育, 且增加根系生长素的合成与转运, 进而影响根系构型。
张淑辉 , 王红 , 王文茹 , 吴雪莲 , 肖元松 , 彭福田 . 蔗糖对桃幼苗生长发育及其SnRK1酶活性的影响[J]. 植物学报, 2019 , 54(6) : 744 -752 . DOI: 10.11983/CBB19032
This study analyzed the SnRK1 activity and seedling growth and development in response to different concentrations and durations of exogenous sucrose in peach (Prunus persica), thus providing theoretical basis and technical support for fruit production. The data showed that 5% sucrose treatment greatly enhanced the SnRK1 activity, which was continuously increased within a certain period of treatment. Compared with water and mannitol controls, 5% sucrose treatment significantly increased the contents of soluble sugar, starch, and chlorophyll, the biomass of aboveground and underground, and the net photosynthetic rate. Examination of root configuration showed that 5% sucrose significantly increased root surface area, root volume, and number of lateral roots, and promoted root thickening and elongation. qRT-PCR analysis showed that exogenous sucrose promoted auxin synthesis and transport in roots. In conclusion, application of certain concentration of sucrose promotes SnRK1 activity, carbon metabolism, and plant growth and development, increases root auxin synthesis and transport and affects root configuration in peach.

Key words: peach; SnRK1; sucrose; auxin; root configuration
| [1] | 董栓泉, 熊茜, 王春幸, 王鸿飞, 邵兴锋, 李和生, 许凤 ( 2016). 蔗糖在延缓青花菜黄化过程中维持其能量和抗氧化力. 园艺学报 43, 1825-1833. |
| [2] | 郭承彬, 董凤丽, 吴明阳 ( 2018). 果树根系生长发育的研究进展及调控应用. 现代园艺 ( 21), 15-17. |
| [3] | 刘丽萍, 臧小云, 袁巧云, 蔡庆生 ( 2006). 外源蔗糖对盐胁迫荞麦幼苗根系生长的缓解效应. 植物生理学通讯 42, 847-850. |
| [4] | 罗静静, 张亚飞, 彭妍, 彭福田, 赵永飞, 于雯 ( 2017). 桃PpSnRK1α在番茄中过表达对营养胁迫下植株生长的影响. 园艺学报 44, 644-652. |
| [5] | 邱念伟, 邓樱 ( 2007). 外源蔗糖显著缓解盐和热胁迫对菠菜PSII颗粒的伤害. 植物学通报 24, 484-489. |
| [6] | 杨凯, 郝锋珍, 续海红, 郭向红, 张鹏飞 ( 2015). 果树根系分布研究进展. 中国农学通报 31(22), 130-135. |
| [7] | 赵世杰, 史国安, 董新纯 (2002). 植物生理学实验指导. 北京: 中国农业科学技术出版社 pp. 84-85, 87-88. |
| [8] | Boriboonkaset T, Theerawitaya C, Yamada N, Pichakum A, Supaibulwatana K, Cha-Um S, Takabe T, Kirdmanee C ( 2013). Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 250, 1157-1167. |
| [9] | Debast S, Nunes-Nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, B?rnke F ( 2011). Altering trehalose-6-phosphate content in transgenic potato tubers growth and alters responsiveness to hormones during sprouting. Plant Physiol 156, 1754-1771. |
| [10] | Emanuelle S, Doblin MS, Stapleton DI, Bacic A, Gooley PR ( 2016). Molecular insights into the enigmatic metabolic regulator, SnRK1. Trends Plant Sci 21, 341-353. |
| [11] | Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K ( 2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9, 436-442. |
| [12] | Geigenberger P, Stitt M, Fernie AR ( 2004). Metabolic control analysis and regulation of the conversion of sucrose to starch in growing potato tubers. Plant Cell Environ 27, 655-673. |
| [13] | Ho SL, Chao YC, Tong WF, Yu SM ( 2001). Sugar coordinately and differentially regulates growth and stress- related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol 125, 877-890. |
| [14] | Jossier M, Bouly JP, Meimoun P, Arjmand A, Lessard P, Hawley S, Hardie DG, Thomas M ( 2009). SnRK1 (SNF1related kinase 1) has a central role in sugar and ABA signaling in Arabidopsis thaliana. Plant J 59, 316-328. |
| [15] | Lastdrager J, Hanson J, Smeekens S ( 2014). Sugar signals and the control of plant growth and development. J Exp Bot 65, 799-807. |
| [16] | Nukarinen E, N?gele T, Pedrotti L, Wurzinger B, Mair A, Landgraf R, B?rnke F, Hanson J, Teige M, Baena- Gonzalez E, Dr?ge-Laser W, Weckwerth W ( 2016). Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci Rep 6, 31697. |
| [17] | Piattoni CV, Bustos DM, Guerrero SA, Iglesias Aá ( 2011). Nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase is phosphorylated in wheat endosperm at serine-404 by an SNF1-related protein kinase allosterically inhibited by ribose-5-phosphate. Plant Physiol 156, 1337-1350. |
| [18] | Rolland F, Baena-Gonzalez E, Sheen J ( 2006). Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Biol 57, 675-709. |
| [19] | Smeekens S, Ma JK, Hanson J, Rolland F ( 2010). Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13, 274-279. |
| [20] | Wurzinger B, Nukarinen E, N?gele T, Weckwerth W, Teige M ( 2018). The SnRK1 kinase as central mediator of energy signaling between different organelles. Plant Physiol 176, 1085-1094. |
| [21] | Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu JQ, Huang JR, Wang GD, Wang JW ( 2013). Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. eLife 2, e00269. |
| [22] | Yu SM, Lo SF, Ho THD ( 2015). Source-sink communication: regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci 20, 844-857. |
| [23] | Zhang YH, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ ( 2009). Inhibition of SNF1-related protein kinase 1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149, 1860-1871. |
/
| 〈 |
|
〉 |