

胞泌复合体在植物中的功能研究进展
收稿日期: 2018-12-04
录用日期: 2019-03-19
网络出版日期: 2019-03-26
基金资助
山东省自然科学基金(ZR2017BC020);山东省自然科学基金(No.ZR2013CL017)
Research Advances in the Functions of Exocyst Complex in Plants
Received date: 2018-12-04
Accepted date: 2019-03-19
Online published: 2019-03-26
囊泡运输是真核生物的一种重要的细胞学活动, 广泛参与多种生物学过程。该过程主要包括囊泡形成、转运、拴系及与目的膜融合4个环节。目前已知9种多蛋白亚基拴系复合体参与不同途径的胞内转运过程, 其中, 胞泌复合体(exocyst complex)介导了运输囊泡与质膜的拴系过程。对胞泌复合体调控机制的认识主要源于酵母(Saccharomyces cerevisiae)和动物细胞的研究。近年来, 植物胞泌复合体的研究也取得了较大进展, 初步结果显示复合体在功能方面具有一些植物特异的调控特点, 广泛参与植物生长发育和逆境响应。该文主要综述胞泌复合体在植物中的研究进展, 旨在为植物胞泌复合体功能研究提供参考。
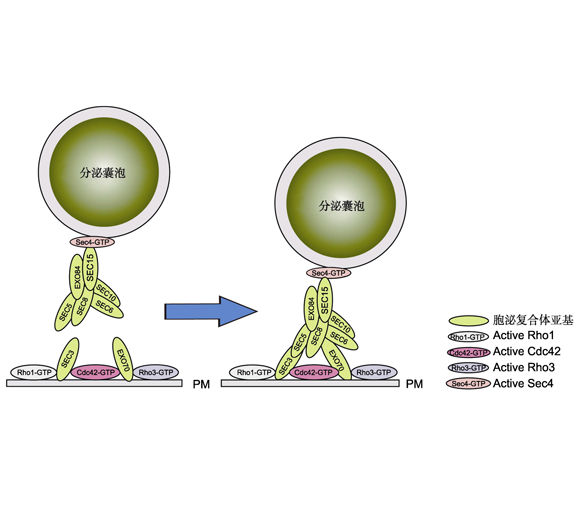
关键词: 囊泡运输; 多蛋白亚基拴系复合体; 胞泌复合体
李彤辉 , 刘晓楠 , 徐静 , 李师鹏 , 蒋苏 . 胞泌复合体在植物中的功能研究进展[J]. 植物学报, 2019 , 54(5) : 642 -651 . DOI: 10.11983/CBB18260
Vesicle trafficking is the biological process involving in budding, translocation, tethering and membrane fusion in all eukaryotic cells. Nine multisubunit tethering complexes (MTCs) are known to play roles in the intracellular transport, and the exocyst complex facilitates the tethering between transport vesicles and the plasma membrane (PM). The regulatory mechanism of the exocyst complex had been extensively studied in yeast and animals, whereas rapid research progress on plant exocyst has been made in recent years. Recent findings show that the plant exocyst complex has unique regulatory characteristics and is widely involved in plant growth, development and stress responses. Here we summarize research progress on the plant exocyst complex to provide a reference for future study of exocyst function in plants.

| 1 | 鲍永美, 王州飞, 张红生 (2005). 植物SNARE蛋白的结构与功能. 植物学通报 22, 715-722. |
| 2 | Adamo JE, Rossi G, Brennwald P (1999). The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol Biol Cell 10, 4121-4133. |
| 3 | Ahmed SM, Nishida-Fukuda H, Li YC, McDonald WH, Gradinaru CC, Macara IG (2018). Exocyst dynamics during vesicle tethering and fusion. Nat Commun 9, 5140. |
| 4 | Bloch D, Pleskot R, Pejchar P, Potocky M, Trpko?ová P, Cwiklik L, Vuka?inovi N, Sternberg H, Yalovsky S, Zársky V (2016). Exocyst SEC3 and phosphoinositides define sites of exocytosis in pollen tube initiation and growth. Plant Physiol 172, 980-1002. |
| 5 | Bonifacino JS, Glick BS (2004). The mechanisms of vesicle budding and fusion. Cell 116, 153-166. |
| 6 | Boyd C, Hughes T, Pypaert M, Novick P (2004). Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol 167, 889-901. |
| 7 | Brunet S, Sacher M (2014). Are all multisubunit tethering complexes bona fide tethers? Traffic 15, 1282-1287. |
| 8 | Cai HQ, Reinisch K, Ferro-Novick S (2007). Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell 12, 671-682. |
| 9 | Chong YT, Gidda SK, Sanford C, Parkinson J, Mullen RT, Goring DR (2010). Characterization of the Arabidopsis thaliana exocyst complex gene families by phylogenetic, expression profiling, and subcellular localization studies. New Phytol 185, 401-419. |
| 10 | Ding Y, Wang J, Lai JHC, Chan VHL, Wang XF, Cai Y, Tan XY, Bao YQ, Xia J, Robinson DG, Jiang LW (2014). Exo70E2 is essential for exocyst subunit recruitment and EXPO formation in both plants and animals. Mol Biol Cell 25, 412-426. |
| 11 | Donovan KW, Bretscher A (2015). Tracking individual secretory vesicles during exocytosis reveals an ordered and regulated process. J Cell Biol 210, 181-189. |
| 12 | Drdová EJ, Klejchová M, Janko K, Hála M, Soukupová H, Cvr?ková F, ?ársky V (2019). Developmental plasticity of Arabidopsis hypocotyl is dependent on exocyst complex function. J Exp Bot 70, 1255-1265. |
| 13 | Drdová EJ, Synek L, Pe?enková T, Hála M, Kulich I, Fowler JE, Murphy AS, ?ársky V (2013). The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J 73, 709-719. |
| 14 | Dubuke ML, Maniatis S, Shaffer SA, Munson M (2015). The exocyst subunit Sec6 interacts with assembled exocytic SNARE complexes. J Biol Chem 290, 28245-28256. |
| 15 | Elias M, Drdova E, Ziak D, Bavlnka B, Hála M, Cvrckova F, Soukupova H, ?ársky V (2003). The exocyst complex in plants. Cell Biol Int 27, 199-201. |
| 16 | Fendrych M, Synek L, Pe?enková T, Toupalová H, Cole R, Drdová E, Nebesárová J, ?edinová M, Hála M, Fowler JE, ?ársky V (2010). The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell 22, 3053-3065. |
| 17 | Finger FP, Hughes TE, Novick P (1998). Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92, 559-571. |
| 18 | Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Schelle RH, Nelson WJ (1998). Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93, 731-740. |
| 19 | Guo JP, Xu CX, Wu D, Zhao Y, Qiu YF, Wang XX, Ouyang YD, Cai BD, Liu X, Jing SL, Shangguan XX, Wang HY, Ma YH, Hu L, Wu Y, Shi SJ, Wang WL, Zhu LL, Xu X, Chen RZ, Feng YQ, Du B, He GC (2018). Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat Genet 50, 297-306. |
| 20 | Guo W, Tamanoi F, Novick P (2001). Spatial regulation of the exocyst complex by Rho1 GTPase. Nat Cell Biol 3, 353-360. |
| 21 | Hála M, Cole R, Synek L, Drdová E, Pe?enková T, Nordheim A, Lamkemeyer T, Madlung J, Hochholdinger F, Fowler JE, ?ársky V (2008). An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20, 1330-1345. |
| 22 | Heider MR, Munson M (2012). Exorcising the exocyst complex. Traffic 13, 898-907. |
| 23 | Hong D, Jeon BW, Kim SY, Hwang JU, Lee Y (2016). The ROP2-RIC7 pathway negatively regulates light-induced stomatal opening by inhibiting exocyst subunit Exo70B1 in Arabidopsis. New Phytol 209, 624-635. |
| 24 | Hong WJ, Lev S (2014). Tethering the assembly of SNARE complexes. Trends Cell Biol 24, 35-43. |
| 25 | Hsu SC, TerBush D, Abraham M, Guo W (2004). The exocyst complex in polarized exocytosis. Int Rev Cytol 233, 243-265. |
| 26 | Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH (1996). The mammalian brain rsec6/8 complex. Neuron 17, 1209-1219. |
| 27 | Kalmbach L, Hématy K, De Bellis D, Barberon M, Fujita S, Ursache R, Daraspe J, Geldner N (2017). Transient cell-specific EXO70A1 activity in the CASP domain and casparian strip localization. Nat Plants 3, 17058. |
| 28 | Kulich I, Cole R, Drdová E, Cvr?ková F, Soukup A, Fowler J, ?ársky V (2010). Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol 188, 615-625. |
| 29 | Kulich I, Pe?enková T, Sekere? J, Smetana O, Fendrych M, Foissner I, H?ftberger M, ?ársky V (2013). Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14, 1155-1165. |
| 30 | Kulich I, Vojtíková Z, Glanc M, Ortmannová J, Rasmann S, ?ársky V (2015). Cell wall maturation of Arabidopsis trichomes is dependent on exocyst subunit EXO70H4 and involves callose deposition. Plant Physiol 168, 120-131. |
| 31 | Lavy M, Bloch D, Hazak O, Gutman I, Poraty L, Sorek N, Sternberg H, Yalovsky S (2007). A novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol 17, 947-952. |
| 32 | Li SP, Chen M, Yu DL, Ren SC, Sun SF, Liu LD, Ketelaar T, Emons AMC, Liu CM (2013). EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25, 1774-1786. |
| 33 | Li SP, van Os GMA, Ren SC, Yu DL, Ketelaar T, Emons AMC, Liu CM (2010). Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol 154, 1819-1830. |
| 34 | Liu DM, Li X, Shen D, Novick P (2018). Two subunits of the exocyst, Sec3p and Exo70p, can function exclusively on the plasma membrane. Mol Biol Cell 29, 736-750. |
| 35 | Luo GZ, Zhang J, Guo W (2014). The role of Sec3p in secretory vesicle targeting and exocyst complex assembly. Mol Biol Cell 25, 3813-3822. |
| 36 | Ma J, Chen J, Wang M, Ren YL, Wang S, Lei CL, Cheng ZJ , Sodmergen (2018). Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J Exp Bot 69, 1051-1064. |
| 37 | Martin-Urdiroz M, Deeks MJ, Horton CG, Dawe HR, Jourdain I (2016). The exocyst complex in health and disease. Front Cell Dev Biol 4, 24. |
| 38 | Mei KR, Guo W (2018). The exocyst complex. Curr Biol 28, R922-R925. |
| 39 | Mei KR, Li Y, Wang SX, Shao GC, Wang J, Ding YH, Luo GZ, Yue P, Liu JJ, Wang XQ, Dong MQ, Wang HW, Guo W (2018). Cryo-EM structure of the exocyst complex. Nat Struct Mol Biol 25, 139-146. |
| 40 | Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA (2002). The exocyst is a Ral effector complex. Nat Cell Biol 4, 66-72. |
| 41 | Munson M, Novick P (2006). The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol 13, 577-581. |
| 42 | Ostertag M, Stammler J, Douchkov D, Eichmann R, Hückelhoven R (2013). The conserved oligomeric golgi complex is involved in penetration resistance of barley to the barley powdery mildew fungus. Mol Plant Pathol 14, 230-240. |
| 43 | Pe?enková T, Hála M, Kulich I, Kocourková D, Drdová E, Fendrych M, Toupalová H, ?ársky V (2011). The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J Exp Bot 62, 2107-2116. |
| 44 | Pleskot R, Cwiklik L, Jungwirth P, ?ársky V, Potocky M (2015). Membrane targeting of the yeast exocyst complex. Biochim Biophys Acta 1848, 1481-1489. |
| 45 | Rawat A, Brej?ková L, Hála M, Cvr?ková F, ?ársky V (2017). The Physcomitrella patens exocyst subunit EXO70.3d has distinct roles in growth and development, and is essential for completion of the moss life cycle. New Phytol 216, 438-454. |
| 46 | Samuel MA, Chong YT, Haasen KE, Aldea-Brydges MG, Stone SL, Goring DR (2009). Cellular pathways regula- ting responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell 21, 2655-2671. |
| 47 | Sekere? J, Pejchar P, ?antr??ek J, Vukasinovi? N, ?ársky V, Potocky M (2017). Analysis of exocyst subunit EXO70 family reveals distinct membrane polar domains in tobacco pollen tubes. Plant Physiol 173, 1659-1675. |
| 48 | Shen D, Yuan H, Hutagalung A, Verma A, Kümmel D, Wu XD, Reinisch K, McNew JA, Novick P (2013). The synaptobrevin homologue Snc2p recruits the exocyst to secretory vesicles by binding to Sec6p. J Cell Biol 202, 509-526. |
| 49 | Sivaram MVS, Saporita JA, Furgason MLM, Boettcher AJ, Munson M (2005). Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry 44, 6302-6311. |
| 50 | Songer JA, Munson M (2009). Sec6p anchors the assembled exocyst complex at sites of secretion. Mol Biol Cell 20, 973-982. |
| 51 | Synek L, Schlager N, Eliá? M, Quentin M, Hauser MT, ?ársky V (2006). AtExo70A1, a member of a family of putative exocyst subunits specifically expanded in land plants, is important for polar growth and plant development. Plant J 48, 54-72. |
| 52 | TerBush DR, Maurice T, Roth D, Novick P (1996). The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J 15, 6483-6494. |
| 53 | TerBush DR, Novick P (1995). Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J Cell Biol 130, 299-312. |
| 54 | Ting AE, Hazuka CD, Hsu SC, Kirk MD, Bean AJ, Scheller RH (1995). rSec6 and rSec8, mammalian homologs of yeast proteins essential for secretion. Proc Natl Acad Sci USA 92, 9613-9617. |
| 55 | Tsuboi T, Ravier MA, Xie H, Ewart MA, Gould GW, Baldwin SA, Rutter GA (2005). Mammalian exocyst complex is required for the docking step of insulin vesicle exocytosis. J Biol Chem 280, 25565-25570. |
| 56 | Tu B, Hu L, Chen WL, Li T, Hu BH, Zheng L, Lv Z, You SJ, Wang YP, Ma BT, Chen XW, Qin P, Li SG (2015). Disruption of OsEXO70A1 causes irregular vascular bundles and perturbs mineral nutrient assimilation in rice. Sci Rep 5, 18609. |
| 57 | Vega IE, Hsu SC (2001). The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neuro Sci 21, 3839-3848. |
| 58 | Wang J, Ding Y, Wang JQ, Hillmer S, Miao YS, Lo SW, Wang XF, Robinson DG, Jiang LW (2010). EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 22, 4009-4030. |
| 59 | Wang Z, Li PF, Yang YJ, Chi YJ, Fan BF, Chen ZX (2016). Expression and functional analysis of a novel group of legume-specific WRKY and EXO70 protein variants from soybean. Sci Rep 6, 32090. |
| 60 | Wen TJ, Hochholdinger F, Sauer M, Bruce W, Schnable PS (2005). The roothairless1 gene of maize encodes a homolog of sec3, which is involved in polar exocytosis. Plant Physiol 138, 1637-1643. |
| 61 | Wu CY, Tan L, van Hooren M, Tan XY, Liu F, Li Y, Zhao YX, Li BX, Rui QC, Munnik T, Bao YQ (2017). Arabidopsis EXO70A1 recruits Patellin3 to the cell membrane independent of its role as an exocyst subunit. J Integr Plant Biol 59, 851-865. |
| 62 | Wu H, Turner C, Gardner J, Temple B, Brennwald P (2010). The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell 21, 430-442. |
| 63 | Wu JD, Tan XY, Wu CY, Cao K, Li Y, Bao YQ (2013). Regulation of cytokinesis by exocyst subunit SEC6 and KEULE in Arabidopsis thaliana. Mol Plant 6, 1863-1876. |
| 64 | Wu SY, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA (2005). Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12, 879-885. |
| 65 | Xiong XH, Xu QW, Huang Y, Singh RD, Anderson R, Leof E, Hu JH, Ling K (2012). An association between type Iγ PI4P 5-kinase and Exo70 directs E-cadherin clustering and epithelial polarization. Mol Biol Cell 23, 87-98. |
| 66 | Yu IM, Hughson FM (2010). Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol 26, 137-156. |
| 67 | Yue P, Zhang YB, Mei KR, Wang SX, Lesigang J, Zhu YY, Dong G, Guo W (2017). Sec3 promotes the initial binary t-SNARE complex assembly and membrane fusion. Nat Commun 8, 14236. |
| 68 | ?ársky V, Cvr?ková F, Potocky M, Hála M (2009). Exocytosis and cell polarity in plants exocyst and recycling domains. New Phytol 183, 255-272. |
| 69 | Zhang XC, Pumplin N, Ivanov S, Harrison MJ (2015). EXO70I is required for development of a sub-domain of the periarbuscular membrane during arbuscular mycorrhizal symbiosis. Curr Biol 25, 2189-2195. |
| 70 | Zhang XY, Bi EF, Novick P, Du LL, Kozminski KG, Lipschutz JH, Guo W (2001). Cdc42 interacts with the exocyst and regulates polarized secretion. J Biol Chem 276, 46745-46750. |
| 71 | Zhang XY, Orlando K, He B, Xi FG, Zhang J, Zajac A, Guo W (2008). Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol 180, 145-158. |
| 72 | Zhang XY, Zajac A, Zhang J, Wang PY, Li M, Murray J, TerBush D, Guo W (2005). The critical role of Exo84p in the organization and polarized localization of the exocyst complex. J Biol Chem 280, 20356-20364. |
| 73 | Zhang Y, Immink R, Liu CM, Emons AM, Ketelaar T (2013). The Arabidopsis exocyst subunit Sec3A is essential for embryo development and accumulates in transient puncta at the plasma membrane. New Phytol 199, 74-88. |
| 74 | Zhu XY, Li SD, Pan SQ, Xin XR, Gu Y (2018). CSI1, PATROL1, and exocyst complex cooperate in delivery of cellulose synthase complexes to the plasma membrane. Proc Natl Acad Sci USA 115, E3578-E3587. |
/
| 〈 |
|
〉 |