

木栓质组成成分、组织化学特性及其生物合成研究进展
# 共同第一作者
收稿日期: 2016-04-01
录用日期: 2016-06-24
网络出版日期: 2017-05-27
基金资助
国家自然科学基金(No.31372113)
Research Progress on Constituents, Histochemical Characteristics and Biosynthesis of Suberin
# Co-first authors
Received date: 2016-04-01
Accepted date: 2016-06-24
Online published: 2017-05-27
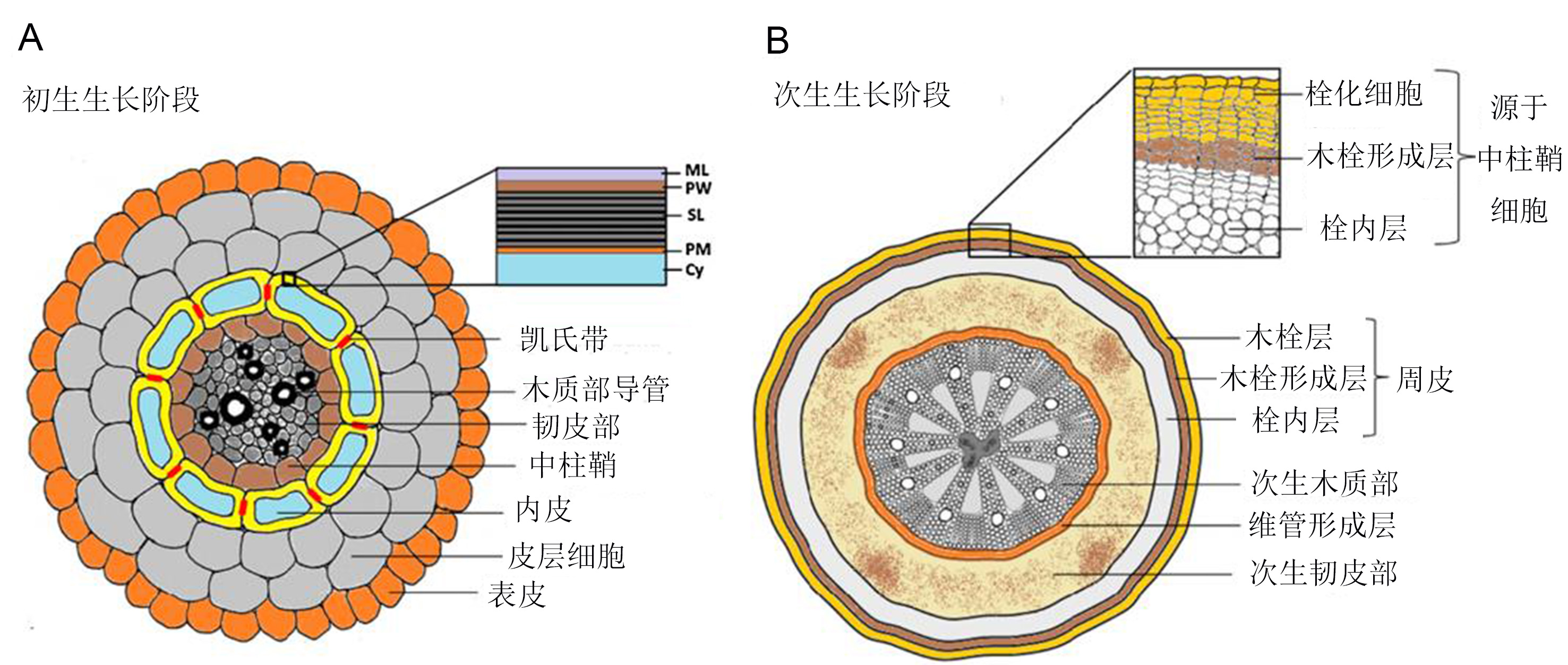
木栓质是一种植物次生代谢产物, 是以甘油为基础的多聚脂类和多聚酚类物质的聚合物, 定位于细胞壁和质膜之间, 主要分布在根皮层和茎的次生边界组织中, 可以降低细胞水分和营养物质的外流, 限制病原体的入侵, 阻碍有毒气体向内扩散。近年来, 随着人们对果蔬贮藏和植物抗性的关注, 对木栓质的研究越来越深入, 尤其是代谢相关酶及基因功能研究。该文系统阐述木栓质组织化学、生物合成及其相关酶和基因的研究动态, 介绍木栓质的生理功能, 总结木栓质组成物质的转运、聚合及其堆积调控等, 以期为木栓质的深入研究和开发利用提供参考。
韩雪源 , 茅林春 . 木栓质组成成分、组织化学特性及其生物合成研究进展[J]. 植物学报, 2017 , 52(3) : 358 -374 . DOI: 10.11983/CBB16068
Located between the cell wall and plasma membrane as a secondary metabolite, suberin typically distributes in rhizodermis and the boundary tissue of stems. Based on glycerol, suberin is a heteropolymer composed of polya- liphatics and polyaromatics, and it could slow the outflow of water and nutrient substance, limit pathogen invasion and prevent toxic gas from diffusing to plants. Recently, with people’s focus on the storage and processing of fruits and vegetables, as well plant resistance, the research in suberin is increasing, especially in the aspects of metabolic enzymes and corresponding genes and the metabolite’s function. In this paper, we elaborate the research progress in suberin histochemistry, the biosynthesis pathway as well related enzymes and genes. We introduce recent advances in the transport of suberin components intracellularly and to the cell wall, polymer assembly, and the regulation of suberin deposition and present the research development of suberin physiological function. This research is expected to provide significant information for further research and application of suberin.

| [1] | Agrawal VP, Kolattukudy P (1977). Biochemistry of suberi- zation ω-hydroxyacid oxidation in enzyme preparations from suberizing potato tuber disks.Plant Physiol 59, 667-672. |
| [2] | Aoki K, Ogata Y, Shibata D (2007). Approaches for extracting practical information from gene co-expression networks in plant biology.Plant Cell Physiol 48, 381-390. |
| [3] | Armstrong J, Armstrong W (2005). Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence.Ann Bot 96, 625-638. |
| [4] | Barrowclough DE, Peterson CA, Steudle E (2000). Radial hydraulic conductivity along developing onion roots.J Exp Bot 51, 547-557. |
| [5] | Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis.PLoS Genet 5, e1000492. |
| [6] | Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB (2007). The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis.Plant Cell 19, 351-368. |
| [7] | Beisson F, Li-Beisson Y, Pollard M (2012). Solving the puzzles of cutin and suberin polymer biosynthesis.Curr Opin Plant Biol 15, 329-337. |
| [8] | Benveniste I, Tijet N, Adas F, Philipps G, Salaün JP, Durst F (1998). CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450-dependent fatty acid omega- hydroxylase. Biochem Biophys Res Commun 243, 688-693. |
| [9] | Bernards MA (2002). Demystifying suberin.Can J Bot 80, 227-240. |
| [10] | Bernards MA, Lopez ML, Zajicek J, Lewis NG (1995). Hydroxycinnamic acid-derived polymers constitute the po- lyaromatic domain of suberin.J Biol Chem 270, 7382-7386. |
| [11] | Bird DA (2008). The role of ABC transporters in cuticular lipid secretion.Plant Sci 174, 563-569. |
| [12] | Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN (2003). A gene expres- sion map of the Arabidopsis root.Science 302, 1956-1960. |
| [13] | Blacklock BJ, Jaworski JG ( 2006). Substrate specificity of Arabidopsis 3-ketoacyl-CoA synthases.Biochem Biophys Res Commun 346, 583-590. |
| [14] | Bomal C, Bedon F, Caron S, Mansfield SD, Levasseur C, Cooke JE, Blais S, Tremblay L, Morency MJ, Pavy N (2008). Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall bio- genesis: a comparative in planta analysis. J Exp Bot 59, 3925-3939. |
| [15] | Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN (2007). A high-resolution root spatiotemporal map reveals dominant expression patterns.Science 318, 801-806. |
| [16] | Brundrett MC, Enstone DE, Peterson CA (1988). A berberine-aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue.Protoplasma 146, 133-142. |
| [17] | Brundrett MC, Kendrick B, Peterson CA (1991). Efficient lipid staining in plant material with Sudan Red 7B or Fluo- ral Yellow 088 in polyethylene glycol-glycerol.Biotech Hi- stochem 66, 111-116. |
| [18] | Choi H, Jin JY, Choi S, Hwang JU, Kim YY, Suh MC, Lee Y (2011). An ABCG/WBC-type ABC transporter is ess- ential for transport of sporopollenin precursors for exine formation in developing pollen.Plant J 65, 181-193. |
| [19] | Clarkson D, Robards A, Stephens J, Stark M (1987). Suberin lamellae in the hypodermis of maize (Zea mays) roots, development and factors affecting the permeability of hypodermal layers. Plant Cell Environ 10, 83-93. |
| [20] | Compagnon V, Diehl P, Benveniste I, Meyer D, Schaller H, Schreiber L, Franke R, Pinot F (2009). CYP86B1 is required for very long chain ω-hydroxyacid and α, ω- dicarboxylic acid synthesis in root and seed suberin polyester.Plant Physiol 150, 1831-1843. |
| [21] | D’Auria JC (2006). Acyltransferases in plants: a good time to be BAHD.Curr Opin Plant Biol 9, 331-340. |
| [22] | De Simone O, Haase K, Müller E, Junk WJ, Hartmann K, Schreiber L, Schmidt W (2003). Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four Amazonian tree species.Plant Physiol 132, 206-217. |
| [23] | DeBolt S, Scheible WR, Schrick K, Auer M, Beisson F, Bischoff V, Bouvier-Navé P, Carroll A, Hematy K, Li Y (2009). Mutations in UDP-glucose: sterol glucosyltrans- ferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds.Plant Physiol 151, 78-87. |
| [24] | Domergue F, Vishwanath SJ, Joubès J, Ono J, Lee JA, Bourdon M, Alhattab R, Lowe C, Pascal S, Lessire R (2010). Three Arabidopsis fatty acyl-coenzyme A reduc- tases, FAR1, FAR4, and FAR5, generate primary fatty al- cohols associated with suberin deposition.Plant Physiol 153, 1539-1554. |
| [25] | Efetova M, Zeier J, Riederer M, Lee CW, Stingl N, Mueller M, Hartung W, Hedrich R, Deeken R (2007). A central role of abscisic acid in drought stress protection of Agro- bacterium-induced tumors on Arabidopsis. Plant Physiol 145, 853-862. |
| [26] | Enstone DE, Peterson CA, Ma F (2003). Root endodermis and exodermis: structure, function, and responses to the environment.J Plant Growth Regul 21, 335-351. |
| [27] | Franke R, Briesen I, Wojciechowski T, Faust A, Yephr- emov A, Nawrath C, Schreiber L (2005). Apoplastic polyesters in Arabidopsis surface tissues—a typical sub- erin and a particular cutin.Phytochemistry 66, 2643-2658. |
| [28] | Franke R, Höfer R, Briesen I, Emsermann M, Efremova N, Yephremov A, Schreiber L (2009). The DAISY gene from Arabidopsis encodes a fatty acid elongase con- densing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J 57, 80-95. |
| [29] | Franke R, Schreiber L (2007). Suberin—a biopolyester forming apoplastic plant interfaces.Curr Opin Plant Biol 10, 252-259. |
| [30] | Franke RB, Dombrink I, Schreiber L (2012). Suberin goes genomics: use of a short living plant to investigate a long lasting polymer.Front Plant Sci 3, 1-8. |
| [31] | Gandini A (2008). Polymers from renewable resources: a challenge for the future of macromolecular materials.Ma- cromolecules 41, 9491-9504. |
| [32] | Garthwaite AJ, Armstrong W, Colmer TD (2008). Assess- ment of O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytol 179, 405-416. |
| [33] | Girard AL, Mounet F, Lemaire-Chamley M, Gaillard C, Elmorjani K, Vivancos J, Runavot JL, Quemener B, Petit J, Germain V (2012). Tomato GDSL1 is required for cutin deposition in the fruit cuticle.Plant Cell 24, 3119-3134. |
| [34] | Gou JY, Yu XH, Liu CJ (2009). A hydroxycinnamoyltransfe- rase responsible for synthesizing suberin aromatics in Arabidopsis.Proc Natl Acad Sci USA 106, 18855-18860. |
| [35] | Graça J (2010). Hydroxycinnamates in suberin formation.Phytochem Rev 9, 85-91. |
| [36] | Graça J, Pereira H (1997). Cork suberin: a glyceryl based polyester.Holzforschung 51, 225-234. |
| [37] | Graça J, Pereira H (2000). Methanolysis of bark suberins: analysis of glycerol and acid monomers.Phytochem Anal 11, 45-51. |
| [38] | Graça J, Santos S (2007). Suberin: a biopolyester of plants’ skin.Macromol Biosci 7, 128-135. |
| [39] | Himmelbach A, Yang Y, Grill E (2003). Relay and control of abscisic acid signaling.Curr Opin Plant Biol 6, 470-479. |
| [40] | Höfer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R (2008). The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. J Exp Bot 59, 2347-2360. |
| [41] | Jessen D, Olbrich A, Knüfer J, Krüger A, Hoppert M, Polle A, Fulda M (2011). Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis.Plant J 68, 715-726. |
| [42] | Joubès J, Raffaele S, Bourdenx B, Garcia C, Laroche- Traineau J, Moreau P, Domergue F, Lessire R (2008). The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression pro- filing. Plant Mol Biol 67, 547-566. |
| [43] | Kandel S, Sauveplane V, Olry A, Diss L, Benveniste I, Pinot F (2006). Cytochrome P450-dependent fatty acid hydroxylases in plants.Phytochem Rev 5, 359-372. |
| [44] | Kato M, Hayakawa Y, Hyodo H, Ikoma Y, Yano M (2000). Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol 41, 440-447. |
| [45] | Kesanakurti D, Kolattukudy PE, Kirti PB (2012). Fruit- specific overexpression of wound-induced tap1 under E8 promoter in tomato confers resistance to fungal patho- gens at ripening stage.Physiol Plant 146, 136-148. |
| [46] | Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007). The AtGenExpress global stress expre- ssion data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses.Plant J 50, 347-363. |
| [47] | Kolattukudy P (1971). Enzymatic synthesis of fatty alcohols in Brassica oleracea. Arch Biochem Biophys 142, 701-709. |
| [48] | Kolattukudy PE (1981). Structure, biosynthesis, and biode- gradation of cutin and suberin.Annu Rev Plant Physiol 32, 539-567. |
| [49] | Kolattukudy PE (2001). Polyesters in higher plants. In: Biopolyesters. Berlin Heidelberg: Springer Press. pp. 1-49. |
| [50] | Kotula L, Ranathunge K, Schreiber L, Steudle E (2009). Functional and chemical comparison of apoplastic barr- iers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot 60, 2155-2167. |
| [51] | Krishnamurthy P, Ranathunge K, Franke R, Prakash H, Schreiber L, Mathew M (2009). The role of root apo- plastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230, 119-134. |
| [52] | Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew M (2011). Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J Exp Bot 62, 4215-4228. |
| [53] | Kumar G, Knowles NR (2003). Wound-induced superoxide production and PAL activity decline with potato tuber age and wound healing ability.Physiol Plant 117, 108-117. |
| [54] | Kumar GM, Iyer S, Knowles NR (2007). Strboh A homolo- gue of NADPH oxidase regulates wound-induced oxida- tive burst and facilitates wound-healing in potato tubers.Planta 227, 25-36. |
| [55] | Kumar GM, Lulai EC, Suttle JC, Knowles NR (2010). Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA.Planta 232, 1433-1445. |
| [56] | Kurdyukov S, Faust A, Nawrath C, Bär S, Voisin D, Efremova N, Franke R, Schreiber L, Saedler H, Métraux JP (2006). The epidermis-specific extracellular BODYGUARD controls cuticle development and morpho- genesis in Arabidopsis.Plant Cell 18, 321-339. |
| [57] | Landgraf R, Smolka U, Altmann S, Eschen-Lippold L, Senning M, Sonnewald S, Weigel B, Frolova N, Stre- hmel N, Hause G (2014). The ABC transporter ABCG1 is required for suberin formation in potato tuber periderm.Plant Cell 26, 3403-3415. |
| [58] | Lasserre E, Jobet E, Llauro C, Delseny M (2008). AtERF38 (At2g35700), an AP2/ERF family transcription factor gene from Arabidopsis thaliana, is expressed in sp- ecific cell types of roots, stems and seeds that undergo suberization. Plant Physiol Biochem 46, 1051-1061. |
| [59] | Lee SB, Jung SJ, Go YS, Kim HU, Kim JK, Cho HJ, Park OK, Suh MC (2009). Two Arabidopsis 3-ketoacyl CoA synthase genes,KCS20 and KCS2/DAISY, are func- tionally redundant in cuticular wax and root suberin bio- synthesis, but differentially controlled by osmotic stress. Plant J 60, 462-475. |
| [60] | Leide J, Hildebrandt U, Hartung W, Riederer M, Vogg G (2012). Abscisic acid mediates the formation of a suber- ized stem scar tissue in tomato fruits.New Phytol 194, 402-415. |
| [61] | Li Y, Beisson F, Koo AJ, Molina I, Pollard M, Ohlrogge J (2007). Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like mo- nomers.Proc Natl Acad Sci USA 104, 18339-18344. |
| [62] | Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, DeBono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu CC, Zallot R, Ohlrogge J (2013). Acyl-lipid metabolism.The Arabidopsis Book 11, e0161. |
| [63] | Lotfy S, Negrel J, Javelle F (1994). Formation of ω-feruloy- loxypalmitic acid by an enzyme from wound-healing pota- to tuber discs.Phytochemistry 35, 1419-1424. |
| [64] | Lü S, Song T, Kosma DK, Parsons EP, Rowland O, Jenks MA (2009). Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlap- ping functions with LACS2 in plant wax and cutin synthe- sis. Plant J 59, 553-564. |
| [65] | Lulai EC, Corsini DL (1998). Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tube- rosum L.) wound-healing. Physiol Mol Plant Pathol 53, 209-222. |
| [66] | Lulai EC, Suttle JC (2004). The involvement of ethylene in wound-induced suberization of potato tuber (Solanum tu- berosum L.): a critical assessment. Postharvest Biol Te- chnol 34, 105-112. |
| [67] | Lulai EC, Suttle JC, Pederson SM (2008). Regulatory involvement of abscisic acid in potato tuber wound-hea- ling.J Exp Bot 59, 1175-1186. |
| [68] | Lux A, Šottníková A, Opatrna J, Greger M (2004). Differ- ences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol Plant 120, 537-545. |
| [69] | Machado A, Pereira H, Teixeira RT (2013). Anatomy and development of the endodermis and phellem of Quercus suber L. roots. Microsc Microanal 19, 525-534. |
| [70] | Matsuda F, Morino K, Miyashita M, Miyagawa H (2003). Metabolic flux analysis of the phenylpropanoid pathway in wound-healing potato tuber tissue using stable isotope- labeled tracer and LC-MS spectroscopy.Plant Cell Phy- siol 44, 510-517. |
| [71] | Metz JG, Pollard MR, Anderson L, Hayes TR, Lassner MW (2000). Purification of a jojoba embryo fatty acyl- coenzyme A reductase and expression of its cDNA in high erucic acid rapeseed.Plant Physiol 122, 635-644. |
| [72] | Meyer CJ, Seago JL, Peterson CA (2009). Environmental effects on the maturation of the endodermis and multi- seriate exodermis of Iris germanica roots. Ann Bot 103, 687-702. |
| [73] | Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005). The NAC transcription factors NST1 and NST2 of Arabi- dopsis regulate secondary wall thickenings and are required for anther dehiscence.Plant Cell 17, 2993-3006. |
| [74] | Molina I, Bonaventure G, Ohlrogge J, Pollard M (2006). The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67, 2597-2610. |
| [75] | Molina I, Li-Beisson Y, Beisson F, Ohlrogge JB, Pollard M (2009). Identification of an Arabidopsis feruloyl-coen- zyme A transferase required for suberin synthesis.Plant Physiol 151, 1317-1328. |
| [76] | Obayashi T, Hayashi S, Saeki M, Ohta H, Kinoshita K (2009). ATTED-II provides coexpressed gene networks for Arabidopsis.Nucleic Acids Res 37, D987-D991. |
| [77] | Obayashi T, Kinoshita K, Nakai K, Shibaoka M, Hayashi S, Saeki M, Shibata D, Saito K, Ohta H (2007). AT- TED-II: a database of co-expressed genes and cis elements for identifying co-regulated gene groups in Arabidopsis. Nucleic Acids Res 35, D863-D869. |
| [78] | Olson DA, Sheares VV (2006). Preparation of unsaturated linear aliphatic polyesters using condensation polymeri- zation.Macromolecules 39, 2808-2814. |
| [79] | Olsson A, Lindström M, Iversen T (2007). Lipase-cataly- zed synthesis of an epoxy-functionalized polyester from the suberin monomer cis-9, 10-epoxy-18-hydroxyoctade- canoic acid. Biomacromolecules 8, 757-760. |
| [80] | Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, Schreiber L, Chory J, Aharoni A (2007). The Arabidopsis DESPERADO/AtWBC11 trans- porter is required for cutin and wax secretion.Plant Phy- siol 145, 1345-1360. |
| [81] | Panikashvili D, Shi JX, Bocobza S, Franke RB, Schreiber L, Aharoni A (2010). The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive or- gans and suberin in roots.Mol Plant 3, 563-575. |
| [82] | Paul S, Gable K, Beaudoin F, Cahoon E, Jaworski J, Napier JA, Dunn TM (2006). Members of the Arabidopsis FAE1-like 3-ketoacyl-CoA synthase gene family substitute for the Elop proteins of Saccharomyces cerevisiae. J Biol Chem 281, 9018-9029. |
| [83] | Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004). Plant cuti- cular lipid export requires an ABC transporter.Science 306, 702-704. |
| [84] | Pinot F, Beisson F (2011). Cytochrome P450 metabolizing fatty acids in plants: characterization and physiological roles.FEBS J 278, 195-205. |
| [85] | Pinto PC, Sousa AF, Silvestre AJ, Neto CP, Gandini A, Eckerman C, Holmbom B (2009). Quercus suber and Betula pendula outer barks as renewable sources of oleo- chemicals: a comparative study. Ind Crops Prod 29, 126-132. |
| [86] | Pollard M, Beisson F, Li Y, Ohlrogge JB (2008). Building lipid barriers: biosynthesis of cutin and suberin.Trends Plant Sci 13, 236-246. |
| [87] | Pulsifer IP, Kluge S, Rowland O (2012). Arabidopsis long- chain acyl-CoA synthetase 1 (LACS1), LACS2, and LA- CS3 facilitate fatty acid uptake in yeast.Plant Physiol Bio- chem 51, 31-39. |
| [88] | Raffaele S, Vailleau F, Léger A, Joubès J, Miersch O, Huard C, Blée E, Mongrand S, Domergue F, Roby D (2008). A MYB transcription factor regulates very-long- chain fatty acid biosynthesis for activation of the hyper- sensitive cell death response in Arabidopsis.Plant Cell 20, 752-767. |
| [89] | Ranathunge K, Schreiber L (2011). Water and solute per- meabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin.J Exp Bot 62, 1961-1974. |
| [90] | Ranathunge K, Schreiber L, Franke R (2011). Suberin research in the genomics era—new interest for an old po- lymer.Plant Sci 180, 399-413. |
| [91] | Ranathunge K, Steudle E, Lafitte R (2005). Blockage of apoplastic bypass-flow of water in rice roots by insoluble salt precipitates analogous to a Pfeffer cell.Plant Cell En- viron 28, 121-133. |
| [92] | Razem FA, Bernards MA (2003). Reactive oxygen species production in association with suberization: evidence for an NADPH-dependent oxidase.J Exp Bot 54, 935-941. |
| [93] | Rowland O, Domergue F (2012). Plant fatty acyl reduc- tases: enzymes generating fatty alcohols for protective layers with potential for industrial applications.Plant Sci 193, 28-38. |
| [94] | Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L (2006). CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis.Plant Physiol 142, 866-877. |
| [95] | Schmutz A, Buchala AJ, Ryser U (1996). Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-asso- ciated fatty acid elongases.Plant Physiol 110, 403-411. |
| [96] | Schnurr J, Shockey J (2004). The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle deve- lopment in Arabidopsis. Plant Cell 16, 629-642. |
| [97] | Schnurr JA, Shockey JM, de Boer GJ (2002). Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arab- idopsis.Plant Physiol 129, 1700-1709. |
| [98] | Schreiber L (2010). Transport barriers made of cutin, suberin and associated waxes.Trends Plant Sci 15, 546-553. |
| [99] | Schreiber L, Franke R, Hartmann K (2005a). Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peri- dermal transpiration. Planta 220, 520-530. |
| [100] | Schreiber L, Franke R, Hartmann KD, Ranathunge K, Steudle E (2005b). The chemical composition of suberin in apoplastic barriers affects radial hydraulic conductivity differently in the roots of rice (Oryza sativa L. cv. IR64) and corn(Zea mays L. cv. Helix). J Exp Bot 56, 1427-1436. |
| [101] | Schreiber L, Franke R, Lessire R (2005c). Biochemical characterization of elongase activity in corn (Zea mays L.) roots. Phytochemistry 66, 131-138. |
| [102] | Schreiber L, Hartmann K, Skrabs M, Zeier J (1999). Apoplastic barriers in roots: chemical composition of end- odermal and hypodermal cell walls.J Exp Bot 50, 1267-1280. |
| [103] | Schuler MA, Duan H, Bilgin M, Ali S (2006). Arabidopsis cytochrome P450s through the looking glass: a window on plant biochemistry.Phytochem Rev 5, 205-237. |
| [104] | Serra O, Hohn C, Franke R, Prat S, Molinas M, Figueras M (2010). A feruloyl transferase involved in the bio- synthesis of suberin and suberin-associated wax is re- quired for maturation and sealing properties of potato periderm.Plant J 62, 277-290. |
| [105] | Serra O, Soler M, Hohn C, Franke R, Schreiber L, Prat S, Molinas M, Figueras M (2009a). Silencing of StKCS6 in potato periderm leads to reduced chain lengths of suberin and wax compounds and increased peridermal transpir- ation. J Exp Bot 60, 697-707. |
| [106] | Serra O, Soler M, Hohn C, Sauveplane V, Pinot F, Franke R, Schreiber L, Prat S, Molinas M, Figueras M (2009b).CYP86A33-targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae, and imp- airs the periderm’s water barrier function. Plant Physiol 149, 1050-1060. |
| [107] | Shockey JM, Fulda MS (2003). Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogen- etic and biochemical analysis reveals a new class of acyl-coenzyme A synthetases.Plant Physiol 132, 1065-1076. |
| [108] | Soler M, Serra O, Molinas M, García-Berthou E, Caritat A, Figueras M (2008). Seasonal variation in transcript abun- dance in cork tissue analyzed by real time RT-PCR.Tree Physiol 28, 743-751. |
| [109] | Soler M, Serra O, Molinas M, Huguet G, Fluch S, Figu- eras M (2007). A genomic approach to suberin biosynthe- sis and cork differentiation.Plant Physiol 144, 419-431. |
| [110] | Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O (2007). Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phr- agmites australis and Glyceria maxima. New Phytol 173, 264-278. |
| [111] | Soukup A, Votrubová O, Čížková H (2002). Development of anatomical structure of roots of Phragmites australis. New Phytol 153, 277-287. |
| [112] | Stark RE, Sohn W, Pacchiano Jr RA, Al-Bashir M, Garbow JR (1994). Following suberization in potato wound periderm by histochemical and solid-state 13C nuclear magnetic resonance methods.Plant Physiol 104, 527-533. |
| [113] | Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F (2005). Cuticular lipid composi- tion, surface structure, and gene expression in Arabi- dopsis stem epidermis.Plant Physiol 139, 1649-1665. |
| [114] | Tamagnone L, Merida A, Parr A, Mackay S, Culianez- Macia FA, Roberts K, Martin C (1998). The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in trans- genic tobacco. Plant Cell 10, 135-154. |
| [115] | Tao X, Mao L, Li J (2016). Abscisic acid mediates wound- healing in harvested tomato fruit.Postharvest Biol Tech- nol 118, 128-133. |
| [116] | Thomas R, Fang X, Ranathunge K, Anderson TR, Peterson CA, Bernards MA (2007). Soybean root su- berin: anatomical distribution, chemical composition, and relationship to partial resistance to Phytophthora sojae. Plant Physiol 144, 299-311. |
| [117] | Trenkamp S, Martin W, Tietjen K (2004). Specific and differential inhibition of very-long-chain fatty acid elong- ases from Arabidopsis thaliana by different herbicides. Proc Natl Acad Sci USA 101, 11903-11908. |
| [118] | Tyerman SD, Bohnert H, Maurel C, Steudle E, Smith J (1999). Plant aquaporins: their molecular biology, bio- physics and significance for plant water relations.J Exp Bot 50, 1055-1071. |
| [119] | Vioque J, Kolattukudy P (1997). Resolution and purification of an aldehyde-generating and an alcohol-generating fatty acyl-CoA reductase from pea leaves (Pisum sativum L.). Arch Biochem Biophys 340, 64-72. |
| [120] | Vishwanath SJ, Delude C, Domergue F (2015). Suberin: biosynthesis, regulation, and polymer assembly of a pro- tective extracellular barrier.Plant Cell Rep 34, 573-586. |
| [121] | Visser E, Colmer T, Blom C, Voesenek L (2000). Changes in growth, porosity, and radial oxygen loss from adven- titious roots of selected mono- and dicotyledonous wetl- and species with contrasting types of aerenchyma.Plant Cell Environ 23, 1237-1245. |
| [122] | Waduwara CI, Walcott SE, Peterson CA (2008). Suberin lamellae of the onion root endodermis: their pattern of development and continuity.Botany 86, 623-632. |
| [123] | Wei H, Persson S, Mehta T, Srinivasasainagendra V, Chen L, Page GP, Somerville C, Loraine A (2006). Transcriptional coordination of the metabolic network in Arabidopsis.Plant Physiol 142, 762-774. |
| [124] | Weng JK, Chapple C (2010). The origin and evolution of lignin biosynthesis.New Phytol 187, 273-285. |
| [125] | White PJ (2001). The pathways of calcium movement to the xylem.J Exp Bot 52, 891-899. |
| [126] | Yadav V, Molina I, Ranathunge K, Castillo IQ, Rothstein SJ, Reed JW (2014). ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidop- sis.Plant Cell 26, 3569-3588. |
| [127] | Yan B, Stark RE (2000). Biosynthesis, molecular structure, and domain architecture of potato suberin: a 13C NMR study using isotopically labeled precursors.J Agric Food Chem 48, 3298-3304. |
| [128] | Yang W, Pollard M, Li-Beisson Y, Beisson F, Feig M, Ohlrogge J (2010). A distinct type of glycerol-3-phos- phate acyltransferase with sn-2 preference and phos- phatase activity producing 2-monoacylglycerol. Proc Natl Acad Sci USA 107, 12040-12045. |
| [129] | Yeats TH, Martin LB, Viart HM, Isaacson T, He Y, Zhao L, Matas AJ, Buda GJ, Domozych DS, Clausen MH (2012). The identification of cutin synthase: formation of the plant polyester cutin.Nat Chem Biol 8, 609-611. |
| [130] | Zhong R, Demura T, Ye ZH (2006). SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis.Plant Cell 18, 3158-3170. |
| [131] | Zhong R, Lee C, Ye ZH (2010). Functional characterization of poplar wood-associated NAC domain transcription fac- tors.Plant Physiol 152, 1044-1055. |
| [132] | Zhou J, Lee C, Zhong R, Ye ZH (2009). MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabi- dopsis.Plant Cell 21, 248-266. |
| [133] | Zimmermann HM, Hartmann K, Schreiber L, Steudle E (2000). Chemical composition of apoplastic transport bar- riers in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210, 302-311. |
/
| 〈 |
|
〉 |