

植物查尔酮合成酶超基因家族的分子进化
? 共同第一作者
收稿日期: 2013-11-04
录用日期: 2014-02-23
网络出版日期: 2015-04-09
基金资助
山东自然科学基金(No.ZR2012CM024)和中国科学院系统与进化植物学国家重点实验室开放课题(No.LSEB201101)
Molecular Evolution of Chalcone Synthase Gene Superfamily in Plants
? These authors contributed equally to this paper
Received date: 2013-11-04
Accepted date: 2014-02-23
Online published: 2015-04-09
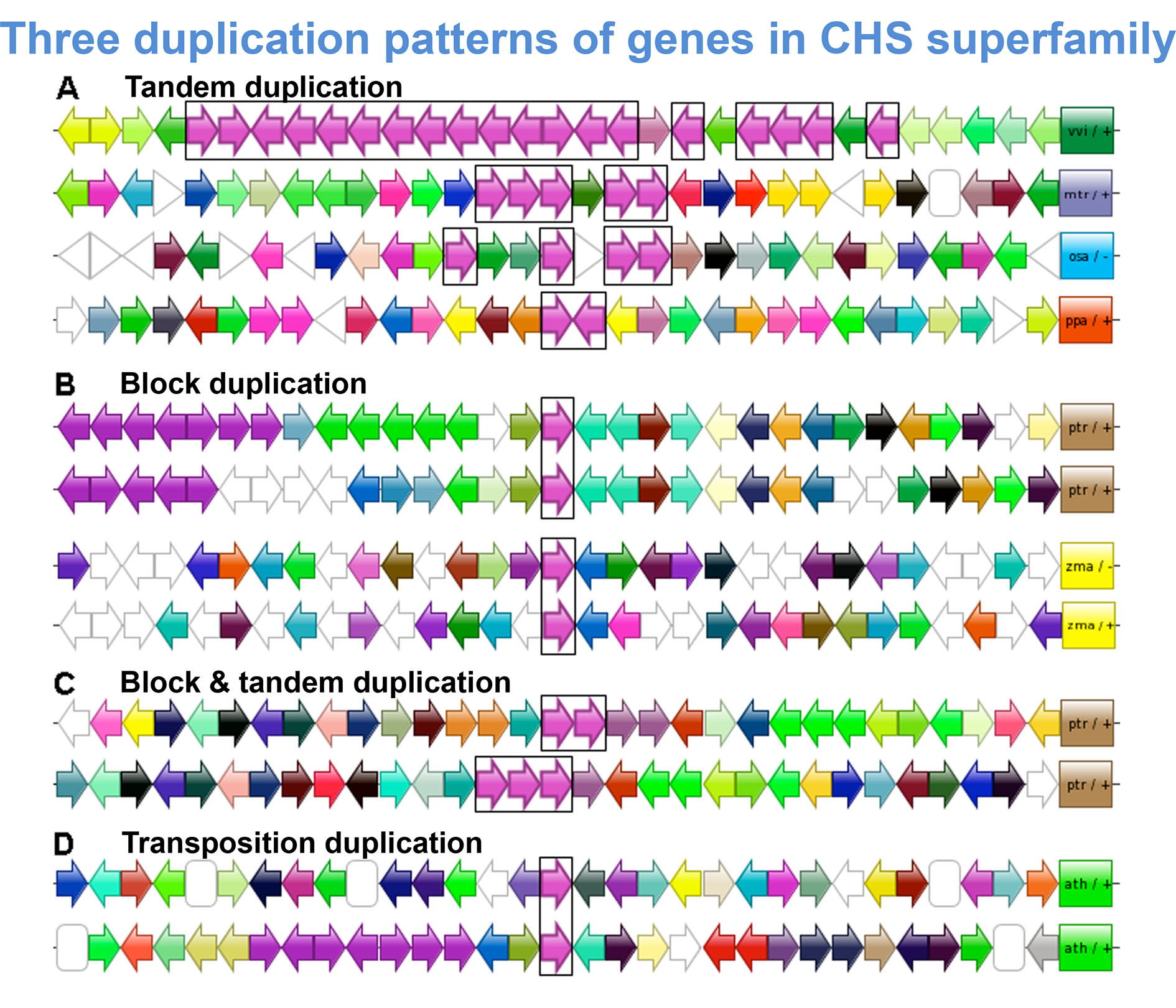
查尔酮合成酶(CHS)超基因家族又称为植物类型III聚酮合酶超基因家族, 其编码酶通过催化和合成一系列结构多样及生理活性各异的次生代谢物, 在植物生长发育和适应环境的过程中扮演着重要角色。为全面了解CHS超基因家族在植物中的进化规律, 重建其进化历史, 该研究利用14种具有全基因组数据的代表植物, 通过生物信息学手段, 深入挖掘和分析了不同植物类群基因组中查尔酮合成酶超基因家族的成员构成, 推测了其可能的扩增机制和功能分歧, 并探讨了该超基因家族在植物中的总体进化趋势。结果共识别144条具有表达信息的同源序列, 它们全部来自9种陆生植物的基因组, 藻类植物基因组中没有发现相关序列。系统发育和进化分析表明, CHS超基因家族的起源古老, 它们可能为适应复杂的生态环境而出现在早期的陆生植物中, 之后在长期的进化过程中不断发生谱系的特异扩张和拷贝丢失, 最后通过功能分歧的形式在不同植物类群中被分别固定。此外, 进化检验也显示, 尽管CHS超基因家族内部发生了多样的遗传改变, 但整个超基因家族仍处于强烈的纯化选择之下, 并且个体基因中也无任何单氨基酸位点受到正向选择的影响。
包颖 , 郭昌锋 , 刘梅 , 陈少华 . 植物查尔酮合成酶超基因家族的分子进化[J]. 植物学报, 2015 , 50(1) : 55 -71 . DOI: 10.3724/SP.J.1259.2015.00055
The chalcone synthase (CHS) gene superfamily, also known as plant-specific type III polyketide synthase gene superfamily, encodes many important enzymes that can catalyze and synthesize various plant secondary metabolites with diverse structures and different biological activities. These metabolites play key roles in plant growth, reproduction, and plant adaptation to the environment. To fully understand the basic evolutionary rules of the CHS gene superfamily in plants and reconstruct its evolutionary history, we performed bioinformatics analysis of CHS genes in 14 plant species with whole-genome data. We performed a BLAST search to identify the gene members of the CHS superfamily. The possible expansion mechanisms and functional divergences of the members were characterized, and the evolution- ary trend of the superfamily was explored. We identified 144 genes with expression information; all are expressed in 9 land plants but not 5 algae. Phylogenetic analysis revealed that the CHS gene superfamily had an ancient origin and complicated evolutionary history. It probably appeared in early terrestrial plants to adapt to the complex environment, then experienced lineage-specific expansions or gene loss during evolution, and finally was fixed in different plant taxa through functional divergences. In addition, evolutionary testing showed that despite diverse genetic differentiation within the CHS superfamily, the whole superfamily was still filtered by strong purifying selection and no single amino acid site within an individual gene was affected by positive selection.

| 1 | Abe I, Morita H (2010). Structure and function of the chalcone synthase superfamily of plant type III poly- ketide synthases. Nat Prod Rep 27, 809-838. |
| 2 | Austin MB, Noel JP (2003). The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20, 79-110. |
| 3 | Baerson SR, Schröder J, Cook D, Rimando AM, Pan ZQ, Dayan FE, Noonan BP, Duke SO (2010). Alkylresorcinol biosynthesis in plants: new insights from an ancient enzyme family? Plant Signal Behav 5, 1286-1289. |
| 4 | Bowers JE, Chapman BA, Rong JK, Paterson AH (2003). Unravelling angiosperm genome evolution by phylo- genetic analysis of chromosomal duplication events.Nature 422, 433-438. |
| 5 | Christensen AB, Gregersen PL, Schröder J, Collinge DB (1998). A chalcone synthase with an unusual substrate preference is expressed in barley leaves in response to UV light and pathogen attack. Plant Mol Biol 37, 849-857. |
| 6 | Cook D, Rimando AM, Clemente TE, Schröder J, Dayan FE, Nanayakkara NP, Pan ZQ, Noonan BP, Fishbein M, Abe I, Duke SO, Baerson SR (2010). Alkylresorcinol synthases expressed in Sorghum bicolor root hairs play an essential role in the biosynthesis of the allelopathic benzoquinone sorgoleone. Plant Cell 22, 867-887. |
| 7 | Criscuolo A (2011). MorePhyML: improving the phylo- genetic tree space exploration with PhyML 3.Mol Phylo- genet Evol 61, 944-948. |
| 8 | Darriba D, Taboada GL, Doallo R, Posada D (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27, 1164-1165. |
| 9 | Dobritsa AA, Lei Z, Nishikawa S, Urbanczyk-Wochniak E, Huhman DV, Preuss D, Sumner LW (2010). LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis. Plant Physiol 153, 937-955. |
| 10 | Durbin ML, Learn GH Jr, Huttley GA, Clegg MT (1995). Evolution of the chalcone synthase gene family in the genus Ipomoea. Proc Natl Acad Sci USA 92, 3338-3342. |
| 11 | Durbin ML, McCaig B, Clegg MT (2000). Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol Biol 42, 79-92. |
| 12 | Ferrer JL, Austin MB, Stewart C Jr, Noel JP (2008). Structure and function of enzymes involved in the bio- synthesis of phenylpropanoids.Plant Physiol Bioch 46, 356-370. |
| 13 | Fliegmann J, Schröder G, Schanz S, Britsch L, Schröder J (1992). Molecular analysis of chalcone and dihydro- pinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol Biol 18, 489-503. |
| 14 | Franken P, Niesbach-Klösgen U, Weydemann U, Maré- chal-Drouard L, Saedler H, Wienand U (1991). The duplicated chalcone synthase genes C2 and Whp (white pollen) of Zea mays are independently regulated; evid- ence for translational control of Whp expression by the anthocyanin intensifying gene in. EMBO J 10, 2605-2612. |
| 15 | Freeling M (2009). Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu Rev Plant Biol 60, 433-453. |
| 16 | Fukada-Tanaka S, Hoshino A, Hisatomi Y, Habu Y, Hasebe M, Iida S (1997). Identification of new chalcone synthase genes for flower pigmentation in the Japanese and common morning glories. Plant Cell Physiol 38, 754-758. |
| 17 | Funa N, Awakawa T, Horinouchi S (2007). Pentaketide resorcylic acid synthesis by type III polyketide synthase from Neurospora crassa.J Biol Chem 282, 14476-14481. |
| 18 | Funa N, Ozawa H, Hirata A, Horinouchi S (2006). Phenolic lipid synthesis by type III polyketide synthases is essential for cyst formation in Azotobacter vinelandii. Proc Natl Acad Sci USA 103, 6356-6361. |
| 19 | Gross F, Luniak N, Perlova O, Gaitatzis N, Jenke- Kodama H, Gerth K, Gottschalk D, Dittmann E, Muller R (2006). Bacterial type III polyketide synthases: phy- logenetic analysis and potential for the production of novel secondary metabolites by heterologous expression in pseudomonads. Arch Microbiol 185, 28-38. |
| 20 | Hanada K, Zou C, Lehti-Shiu MD, Shinozaki K, Shiu SH (2008). Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environ- mental stimuli. Plant Physiol 148, 993-1003. |
| 21 | Hihara Y, Hara C, Uchimiya H (1996). Isolation and characterization of two cDNA clones for mRNAs that are abundantly expressed in immature anthers of rice (Oryza sativa L.). Plant Mol Biol 30, 1181-1193. |
| 22 | Hopwood DA, Sherman DH (1990). Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet 24, 37-66. |
| 23 | Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, Vezzi A, Legeai F, Hugueney P, Dasilva C, Horner D, Mica E, Jublot D, Poulain J, Bruyère C, Billault A, Segurens B, Gouyvenoux M, Ugarte E, Cattonaro F, Anthouard V, Vico V, Del Fabbro C, Alaux M, Di Gaspero G, Dumas V, Felice N, Paillard S, Juman I, Moroldo M, Scalabrin S, Canaguier A, Le Clainche I, Malacrida G, Durand E, Pesole G, Laucou V, Chatelet P, Merdinoglu D, Delledonne M, Pezzotti M, Lecharny A, Scarpelli C, Artiguenave F, Pè ME, Valle G, Mor- gante M, Caboche M, Adam-Blondon AF, Weissenbach J, Quétier F, Wincker P (2007). The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449, 463-467. |
| 24 | Jez JM, Ferrer JL, Bowman ME, Austin MB, Schröder J, Dixon RA, Noel JP (2001). Structure and mechanism of chalcone synthase-like polyketide synthases. J Ind Mi- crobiol Biot 27, 393-398. |
| 25 | Jiang C, Kim SY, Suh DY (2008). Divergent evolution of the thiolase superfamily and chalcone synthase family. Mol Phylogenet Evol 49, 691-701. |
| 26 | Jiang CG, Schommer CK, Kim SY, Suh DY (2006). Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry 67, 2531-2540. |
| 27 | Jiao YN, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang HY, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, de Pamphilis CW (2011). Ancestral polyploidy in seed plants and angio- sperms. Nature 473, 97-100. |
| 28 | Katsuyama Y, Matsuzawa M, Funa N, Horinouchi S (2007).In vitro synthesis of curcuminoids by type III polyketide synthase from Oryza sativa. J Biol Chem 282, 37702-37709. |
| 29 | Kim SS, Grienenberger E, Lallemand B, Colpitts CC, Kim SY, Souza Cde A, Geoffroy P, Heintz D, Krahn D, Kaiser M, Kombrink E, Heitz T, Suh DY, Legrand M, Douglas CJ (2010). LAP6/POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B encode hydro- xyalkyl α-pyrone synthases required for pollen develop- ment and sporopollenin biosynthesis in Arabidopsis tha- liana. Plant Cell 22, 4045-4066. |
| 30 | Kodan A, Kuroda H, Sakai F (2002). A stilbene synthase from Japanese red pine (Pinus densiflora): implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis. Proc Natl Acad Sci USA 99, 3335-3339. |
| 31 | Koduri PK, Gordon GS, Barker EI, Colpitts CC, Ashton NW, Suh DY (2010). Genome-wide analysis of the chal- cone synthase superfamily genes of Physcomitrella patens. Plant Mol Biol 72, 247-263. |
| 32 | Koes RE, Spelt CE, van den Elzen PJ, Mol JNM (1989). Cloning and molecular characterization of the chalcone synthase multigene family of Petunia hybrida. Gene 81, 245-257. |
| 33 | Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007). Clustal W and Clustal X version 2.0. Bio- informatics 23, 2947-2948. |
| 34 | Lynch M (2002). Genomics. Gene duplication and evolution. Science 297, 945-947. |
| 35 | Lynch M, Conery JS (2000). The evolutionary fate and consequences of duplicate genes. Science 290, 1151-1155. |
| 36 | Lyons E, Pedersen B, Kane J, Alam M, Ming R, Tang H, Wang X, Bowers J, Paterson A, Lisch D, Freeling M (2008). Finding and comparing syntenic regions among Arabidopsis and the outgroups papaya, poplar, and grape: CoGe with rosids. Plant Physiol 148, 1772-1781. |
| 37 | Miché L, Belkin S, Rozen R, Balandreau J (2003). Rice seedling whole exudates and extracted alkylresorcinols induce stress-response in Escherichia coli biosensors. Environ Microbiol 5, 403-411. |
| 38 | Miyazono K, Um J, Imai FL, Katsuyama Y, Ohnishi Y, Horinouchi S, Tanokura M (2011). Crystal structure of curcuminoid synthase CUS from Oryza sativa. Proteins 79, 669-673. |
| 39 | Morita H, Wanibuchi K, Nii H, Kato R, Sugio S, Abe I (2010). Structural basis for the one-pot formation of the diarylheptanoid scaffold by curcuminoid synthase from Oryza sativa. Proc Natl Acad Sci USA 107, 19778-19783. |
| 40 | Parage C, Tavares R, Réty S, Baltenweck-Guyot R, Poutaraud A, Renault L, Heintz D, Lugan R, Marais GA, Aubourg S, Hugueney P (2012). Structural, func- tional, and evolutionary analysis of the unusually large stilbene synthase gene family in grapevine. Plant Physiol 160, 1407-1419. |
| 41 | Proost S, Fostier J, De Witte D, Dhoedt B, Demeester P, Van de Peer Y, Vandepoele K (2012). i-ADHoRe 3.0-fast and sensitive detection of genomic homology in extremely large data sets. Nucleic Acids Res 40, e11. |
| 42 | Rizzon C, Ponger L, Gaut BS (2006). Striking similarities in the genomic distribution of tandemly arrayed genes in Arabidopsis and rice. PLoS Comput Biol 2, e115. |
| 43 | Ross AB, Shepherd MJ, Schüpphaus M, Sinclair V, Alfaro B, Kamal-Eldin A, Aman P (2003). Alkylre- sorcinols in cereals and cereal products. J Agr Food Chem 51, 4111-4118. |
| 44 | Rubinstein CV, Gerrienne P, de la Puente GS, Astini RA, Steemans P (2010). Early Middle Ordovician evidence for land plants in Argentina (eastern Gondwana). New Phytol 188, 365-369. |
| 45 | Sanderson MJ (2003). Molecular data from 27 proteins do not support a Precambrian origin of land plants.Am J Bot 90, 954-956. |
| 46 | Schanz S, Schröder G, Schröder J (1992). Stilbene synthase from Scots pine (Pinus sylvestris). FEBS Lett 313, 71-74. |
| 47 | Schröder J (2000). The family of chalcone synthase-related proteins functional diversity and evolution.Recent Adv Phytochem 34, 55-89. |
| 48 | Schröder J (1997). A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci 2, 373-378. |
| 49 | Schröder J, Schröder G (1990). Stilbene and chalcone synthases: related enzymes with key functions in plant- specific pathways. Z Naturforsch C 45, 1-8. |
| 50 | Stafford HA (1991). Flavonoid evolution: an enzymic ap- proach. Plant Physiol 96, 680-685. |
| 51 | Suyama M, Torrents D, Bork P (2006). PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34, W609-W612. |
| 52 | Tang H, Bowers JE, Wang X, Paterson AH (2010). Angiosperm genome comparisons reveal early polyploidy in the monocot lineage. Proc Natl Acad Sci USA 107, 472-477. |
| 53 | Tropf S, Karcher B, Schröder G, Schröder J (1995). Reaction mechanisms of homodimeric plant polyketide synthases (stilbene and chalcone synthase). A single active site for the condensing reaction is sufficient for synthesis of stilbenes, chalcones, and 6'-deoxychalcon- es.J Biol Chem 270, 7922-7928. |
| 54 | Tropf S, Lanz T, Rensing SA, Schröder J, Schröder G (1994). Evidence that stilbene synthases have developed from chalcone synthases several times in the course of evolution. J Mol Evol 38, 610-618. |
| 55 | Vannozzi A, Dry IB, Fasoli M, Zenoni S, Lucchin M (2012). Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol 12, 130. |
| 56 | Vision TJ, Brown DG, Tanksley SD (2000). The origins of genomic duplications in Arabidopsis. Science 290, 2114-2117. |
| 57 | Walden AR, Walter C, Gardner RC (1999). Genes expressed in Pinus radiata male cones include homologs to anther-specific and pathogenesis response genes. Plant Physiol 121, 1103-1116. |
| 58 | Wang WK, Schaal BA, Chiou YM, Murakami N, Ge XJ, Huang CC, Chiang TY (2007). Diverse selective modes among orthologs/paralogs of the chalcone synthase (Chs) gene family of Arabidopsis thaliana and its relative A. halleri ssp. gemmifera. Mol Phylogenet Evol 44, 503-520. |
| 59 | Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ (2009). Jalview Version 2―a multiple sequ- ence alignment editor and analysis workbench. Bioinfor- matics 25, 1189-1191. |
| 60 | Yamaguchi T, Kurosaki F, Suh DY, Sankawa U, Nishioka M, Akiyama T, Shibuya M, Ebizuka Y (1999). Cross- reaction of chalcone synthase and stilbene synthase overexpressed in Escherichia coli. FEBS Lett 460, 457-461. |
| 61 | Yang ZH (2007). PAML 4: phylogenetic analysis by maximum likelihood.Mol Biol Evol 24, 1586-1591. |
| 62 | Zarnowska ED, Zarnowski R, Kozubek A (2000). Alkyl- resorcinols in fruit pulp and leaves of Ginkgo biloba L. Z Naturforsch C 55, 881-885. |
| 63 | Zhan J (2009). Biosynthesis of bacterial aromatic poly- ketides. Curr Top Med Chem 9, 1958-1610. |
/
| 〈 |
|
〉 |