

水稻水杨酸代谢突变体高通量筛选方法的建立与应用
收稿日期: 2024-09-25
录用日期: 2024-12-14
网络出版日期: 2024-12-24
基金资助
浙江省自然科学基金(LZ23C020001);浙江省自然科学基金(ZCLMS25C1301);国家自然科学基金(31670277)
Establishment and Application of a High-throughput Screening Method for Salicylic Acid Metabolic Mutants in Rice
Received date: 2024-09-25
Accepted date: 2024-12-14
Online published: 2024-12-24
水杨酸(SA)是植物免疫的关键防御信号分子。植物SA的定量分析对于SA代谢途径及其生物学功能研究至关重要。利用高效液相色谱仪(HPLC)和液相-质谱联用仪(LC-MS)测定SA含量是目前常用方法, 但难以实现高通量测定。水稻(Oryza sativa)中SA合成代谢途径目前尚未完全解析, 高效筛选水稻SA相关突变体对于阐明其代谢途径具有重要意义。该文对已有基于SA生物传感菌株Acinetobacter sp. ADPWH_lux估算SA的分析方法进行了改良, 建立了水稻SA高通量估算方法, 简化了样品采集和提取过程, 省去样品称重、组织研磨及离心等耗时步骤, 整个操作流程便捷且高效。同时, 利用已报道的水稻SA代谢相关遗传材料验证了该方法的可行性, 并使用该方法筛选了钴-60诱变的水稻突变体库, 获得一批水稻SA含量发生显著变化的突变体, 采用HPLC法对突变体内源SA进行了验证。该方法可用于SA代谢突变体的遗传筛选及其代谢相关酶鉴定, 对水稻等作物的SA代谢及生物学功能研究具有重要的应用价值。

叶灿 , 姚林波 , 金莹 , 高蓉 , 谭琪 , 李旭映 , 张艳军 , 陈析丰 , 马伯军 , 章薇 , 张可伟 . 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025 , 60(4) : 586 -596 . DOI: 10.11983/CBB24148
INTRODUCTION Salicylic acid (SA) plays an important role in the plant immune system. The quantitative analysis of SA in plants is fundamental to studying SA metabolism and its biological functions. Although high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC/MS) are widely used for SA determination, their low throughput limits their suitability for large-scale analysis. However, the SA biosynthetic pathway in rice is not well understood, highlighting the need for efficient methods to screen SA-related mutants and elucidate SA metabolic pathways.
RATIONALECurrent methods for measuring endogenous SA levels, such as HPLC and LC/MS, involve labor-intensive sample preparation, making them unsuitable for high-throughput analysis. While a lux gene-based SA biosensor has been successfully used in tobacco and Arabidopsis, a reliable and efficient method for SA detection in rice remains unavailable. To address this problem, we optimized sample processing and operational workflows to enable high- throughput SA quantification in rice plants.
RESULTS We developed a streamlined, high-throughput method for SA quantification in rice, eliminating time-consuming steps such as sample weighing, tissue grinding, and centrifugation. This approach significantly simplifies the process while maintaining efficiency and accuracy. We validated the method’s feasibility using published rice SA metabolic mutants. We then applied it to screen a Cobalt-60 induced rice mutant library, identifying mutants with altered SA metabolism. Endogenous SA levels in these mutants were confirmed using HPLC. The results demonstrate the method’s effectiveness in screening SA-related metabolic mutants, providing a valuable tool for studying SA metabolism and its roles in rice and other crops. The method was validated using known SA genetic materials. SA content-altered mutants were successfully isolated for further research.
CONCLUSION This study establishes a rapid and cost-effective method for measuring SA content in rice tissues using the SA biosensor Acinetobacter sp. ADPWH_lux. Given the pivotal role of SA in plant defense, our method adopts streamlined sampling process, requiring only leaf clipping and boiling in LB medium, and dramatically reduces the time and effort associated with tissue collection and processing. This high-throughput approach is well-suited for large-scale screening of greenhouse-grown or hydroponic plants, providing a powerful platform for advancing research on SA metabolism and its biological functions in crops.
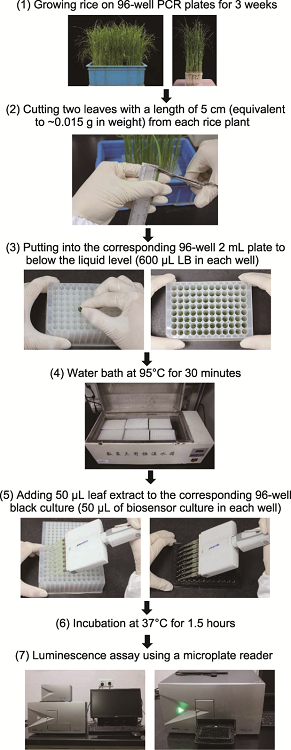
Modified manipulating process for high-throughput determination of salicylic acid (SA) content using SA biosensor Acinetobacter sp. ADPWH_lux strain in rice

| [1] | Aboul-Soud MAM, Cook K, Loake GJ (2004). Measurement of salicylic acid by a high-performance liquid chromatography procedure based on ion-exchange. Chromatographia 59, 129-133. |
| [2] | Ahmad P, Prasad MNV (2012). Abiotic Stress Responses in Plants:Metabolism, Productivity and Sustainability. New York: Springer Press. pp. 312-466. |
| [3] | Cao DD, Chen SY, Qin YB, Wu HP, Ruan GH, Huang YT (2020). Regulatory mechanism of salicylic acid on seed germination under salt stress in kale. Chin Bull Bot 55, 49-61. (in Chinese) |
| 曹栋栋, 陈珊宇, 秦叶波, 吴华平, 阮关海, 黄玉韬 (2020). 水杨酸调控盐胁迫下羽衣甘蓝种子萌发的机理. 植物学报 55, 49-61. | |
| [4] | Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF (2011). Salicylic acid biosynthesis and metabolism. Arabidopsis Book 9, e0156. |
| [5] | Ding PT, Ding YL (2020). Stories of salicylic acid: a plant defense hormone. Trends Plant Sci 25, 549-565. |
| [6] | Ding XY, Zhang J, Su BL, Xu HF (2001). Role of salicylic acid in plant disease resistance. Chin Bull Bot 18, 163-168. (in Chinese) |
| 丁秀英, 张军, 苏宝林, 徐惠风 (2001). 水杨酸在植物抗病中的作用. 植物学通报 18, 163-168. | |
| [7] | Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754-756. |
| [8] | Huang WE, Huang LF, Preston GM, Naylor M, Carr JP, Li YH, Singer AC, Whiteley AS, Wang H (2006). Quantitative in situ assay of salicylic acid in tobacco leaves using a genetically modified biosensor strain of Acinetobacter sp. ADP1. Plant J 46, 1073-1083. |
| [9] | Huang WE, Wang H, Zheng HJ, Huang LF, Singer AC, Thompson I, Whiteley AS (2005). Chromosomally located gene fusions constructed in Acinetobacter sp. ADP1 for the detection of salicylate. Environ Microbiol 7, 1339-1348. |
| [10] | Koo YM, Heo AY, Choi HW (2020). Salicylic acid as a safe plant protector and growth regulator. Plant Pathol J 36, 1-10. |
| [11] | Li C, Zhou L, Zhang YJ, Song FP, Zhang J (2009). Influence of ions conditions on salR gene in Acinetobacter sp. ADP1. Biotechnol Bull (9), 57-63. (in Chinese) |
| 李超, 周琳, 张永军, 宋福平, 张杰 (2009). 离子环境对Acinetobacter sp. ADP1的salR基因活性的影响. 生物技术通报 (9), 57-63. | |
| [12] | Liu SJ, Wu YN, Fang CG, Cui Y, Jiang N, Wang H (2017). Simultaneous determination of 19 plant growth regulator residues in plant-originated foods by QuEChERS and stable isotope dilution-ultra performance liquid chromatography-mass spectrometry. Anal Sci 33, 1047-1052. |
| [13] | Malamy J, Carr JP, Klessig DF, Raskin I (1990). Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250, 1002-1004. |
| [14] | Marek G, Carver R, Ding YZ, Sathyanarayan D, Zhang XD, Mou ZL (2010). A high-throughput method for isolation of salicylic acid metabolic mutants. Plant Methods 6, 21. |
| [15] | Meighen EA (1993). Bacterial bioluminescence: organization, regulation, and application of the lux genes. FASEB J 7, 1016-1022. |
| [16] | Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B (1990). Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250, 1004-1006. |
| [17] | Peng YJ, Yang JF, Li X, Zhang YL (2021). Salicylic acid: biosynthesis and signaling. Annu Rev Plant Biol 72, 761-791. |
| [18] | Rasmussen JB, Hammerschmidt R, Zook MN (1991). Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv. syringae. Plant Physiol 97, 1342-1347. |
| [19] | Rekhter D, Lüdke D, Ding YL, Feussner K, Zienkiewicz K, Lipka V, Wiermer M, Zhang YL, Feussner I (2019). Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365, 498-502. |
| [20] | Rivas-San Vicente M, Plasencia J (2011). Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62, 3321-3338. |
| [21] | Saleem M, Fariduddin Q, Castroverde CDM (2021). Salicylic acid: a key regulator of redox signaling and plant immunity. Plant Physiol Biochem 168, 381-397. |
| [22] | Sanders IO, Smith AR, Hall MA (1989). The measurement of ethylene binding and metabolism in plant tissue. Planta 179, 97-103. |
| [23] | Shields A, Shivnauth V, Castroverde CDM (2022). Salicylic acid and N-hydroxypipecolic acid at the fulcrum of the plant immunity-growth equilibrium. Front Plant Sci 13, 841688. |
| [24] | van Butselaar T, Van den Ackerveken G (2020). Salicylic acid steers the growth-immunity tradeoff. Trends Plant Sci 25, 566-576. |
| [25] | Verberne MC, Brouwer N, Delbianco F, Linthorst HJM, Bol JF, Verpoorte R (2002). Method for the extraction of the volatile compound salicylic acid from tobacco leaf material. Phytochem Anal 13, 45-50. |
| [26] | Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI (2022). Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol 23, 680-694. |
| [27] | Wang YK, Jin GC, Song SY, Jin YJ, Wang XW, Yang SQ, Shen XX, Gan YB, Wang YX, Li R, Liu JX, Hu JP, Pan RH (2024). A peroxisomal cinnamate: CoA ligase-dependent phytohormone metabolic cascade in submerged rice germination. Dev Cell 59, 1363-1378. |
| [28] | White RF (1979). Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology 99, 410-412. |
| [29] | Xu L, Zhao HY, Wang JB, Wang XM, Jia XQ, Wang L, Xu Z, Li RL, Jiang K, Chen ZX, Luo J, Xie XD, Yi KK (2023). AIM1-dependent high basal salicylic acid accumulation modulates stomatal aperture in rice. New Phytol 238, 1420-1430. |
| [30] | Yang CK, Shen SQ, Zhou S, Li YF, Mao YY, Zhou JJ, Shi YH, An LX, Zhou QQ, Peng WJ, Lyu Y, Liu XM, Chen W, Wang SC, Qu LH, Liu XQ, Fernie AR, Luo J (2022). Rice metabolic regulatory network spanning the entire life cycle. Mol Plant 15, 258-275. |
| [31] | Zhang YJ, Yu QL, Gao SL, Yu NN, Zhao L, Wang JB, Zhao JZ, Huang P, Yao LB, Wang M, Zhang KW (2022). Disruption of the primary salicylic acid hydroxylases in rice enhances broad-spectrum resistance against pathogens. Plant Cell Environ 45, 2211-2225. |
| [32] | Zhang YL, Li X (2019). Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol 50, 29-36. |
| [33] | Zhao JZ, Yu NN, Ju M, Fan B, Zhang YJ, Zhu EG, Zhang MY, Zhang KW (2019). ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J Exp Bot 70, 6277-6291. |
/
| 〈 |
|
〉 |