

棉花抗黄萎病相关基因GhDIR1的生物学功能分析
收稿日期: 2024-09-04
录用日期: 2024-10-30
网络出版日期: 2024-11-15
基金资助
农业生物育种重大项目(2023ZD04039);浙江省自然科学基金(LZ23C130004);河北省重点研发计划(21326314D)
Functional Verification of GhDIR1 Gene Against Verticillium Wilt in Cotton
Received date: 2024-09-04
Accepted date: 2024-10-30
Online published: 2024-11-15
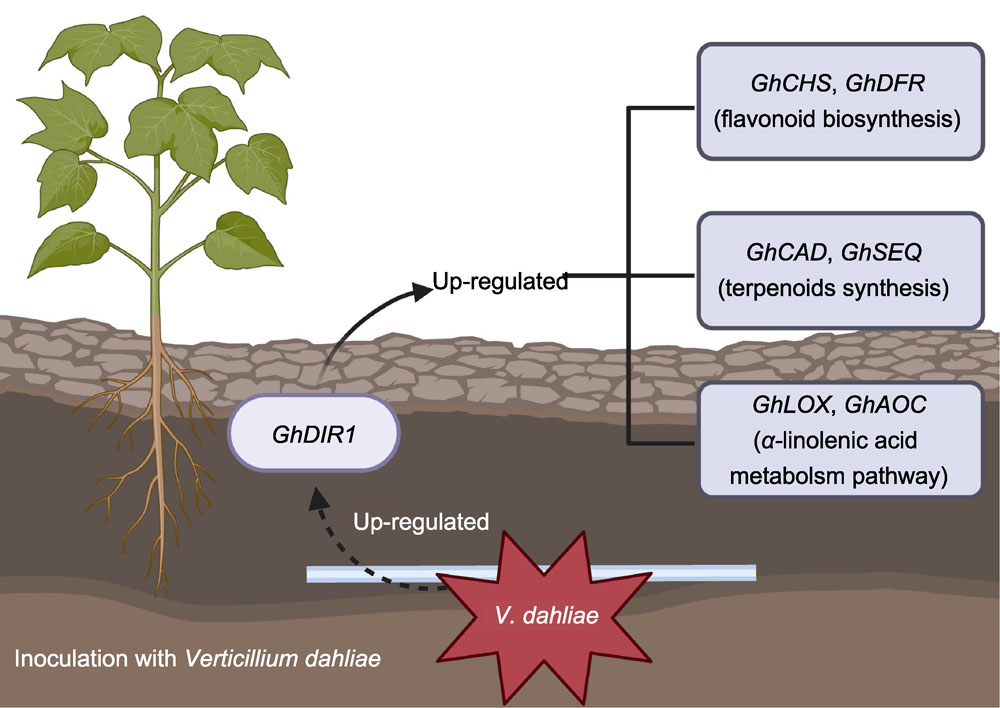
由大丽轮枝菌(Verticillium dahliae)引起的黄萎病是棉花(Gossypium hirsutum)生产中最主要的威胁之一, 其可导致棉花大幅减产和纤维品质严重下降。前期对接种大丽轮枝菌的拟南芥(Arabidopsis thaliana)进行转录组分析, 表明DIR1类蛋白基因AT3G53980.2受病原菌强烈诱导表达。该研究发现, 棉花脂质转移蛋白编码基因GhDIR1 (Gh_A09G180700.1)与AT3G53980.2表现出高度的同源性。生物信息学分析表明, GhDIR1开放阅读框(ORF)为351 bp, 编码116个氨基酸残基。亚细胞定位结果显示GhDIR1定位于细胞膜。分析GhDIR1在大丽轮枝菌V991侵染后的表达模式, 发现其能快速响应大丽轮枝菌侵染。利用病毒诱导的基因沉默(VIGS)技术下调该基因表达后, 棉花对黄萎病菌的抗性显著降低。野生型和GhDIR1沉默植株转录组测序结果表明, 差异表达基因主要在类黄酮生物合成、倍半萜和三萜生物合成以及α-亚麻酸代谢3个途径富集; 同时, 荧光定量PCR结果表明, 3个途径中的6个关键基因(GhCHS、GhDFR、GhCAD、GhSEQ、GhLOX和GhAOC)在GhDIR1沉默植株中均下调表达, 与转录组数据一致。推测GhDIR1可能通过介导类黄酮和萜类化合物的合成途径, 并调节茉莉酸(JA)等植物激素的次级代谢来激活相关信号通路, 进而影响植株抗病性。综上, GhDIR1作为棉花抗黄萎病的正向调控因子, 通过参与多种激素和抗病信号网络调控植物的免疫反应。
黄雨欣 , 谢涛 , 王省芬 , 郭惠明 , 程红梅 , 马伯军 , 陈析丰 , 苏晓峰 . 棉花抗黄萎病相关基因GhDIR1的生物学功能分析[J]. 植物学报, 2025 , 60(5) : 816 -830 . DOI: 10.11983/CBB24135
INTRODUCTION: Verticillium wilt (VW), caused by Verticillium dahliae, severely reduces cotton yield and fiber quality. Previous transcriptomic analysis in V. dahliae-inoculated Arabidopsis thaliana identified the pathogen-induced DIR1-like gene AT3G53980.2. In cotton, we discovered a homologous gene, GhDIR1 (Gh_A09G180700.1), encoding a lipid transfer protein. This study investigates its role in cotton resistance to V. dahliae.
RATIONALE: We characterized GhDIR1’s molecular features, expression patterns under pathogen stress, and functional impact using bioinformatics, subcellular localization, qRT-PCR, and virus-induced gene silencing (VIGS) analyses. Transcriptomic analysis of wild-type and GhDIR1-silenced plants were conducted to unravel downstream regulatory networks, focusing on metabolic pathways linked to plant immunity.
RESULTS: The results showed that GhDIR1 contains a 351 bp ORF encoding 116 amino acids. Subcellular localization confirmed its presence on the cell membrane. qRT-PCR showed rapid induction of GhDIR1 by V. dahliae. Silencing GhDIR1 increased cotton susceptibility to the pathogen. Transcriptomic data revealed that differentially expressed genes in silenced plants were enriched in flavonoid biosynthesis, sesquiterpene/triterpene biosynthesis, and α-linolenic acid metabolism. Key genes (GhCHS, GhDFR, GhCAD, GhSEQ, GhLOX, and GhAOC) in these pathways were downregulated, suggesting impaired synthesis of protective metabolites.
CONCLUSION: It is speculated that GhDIR1 positively regulates cotton resistance to VW by modulating flavonoid and terpenoid biosynthesis and jasmonic acid-related signaling. Its silencing disrupts critical defense pathways, highlighting its role in coordinating immune responses. These findings propose GhDIR1 as a potential target for enhancing disease resistance in cotton.
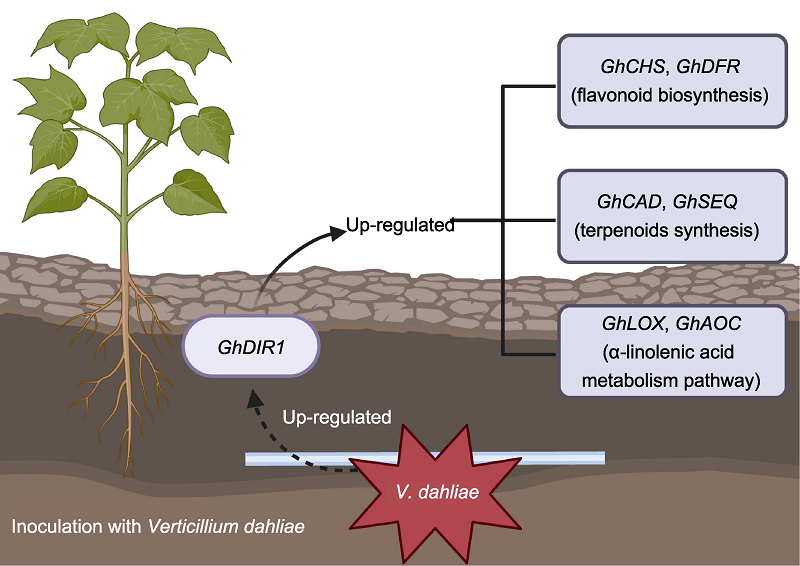
The induced expression pattern of GhDIR1 and related genes after inoculation with Verticillium dahliae.

| [1] | Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16, 3460-3479. |
| [2] | Chen B, Zhang Y, Yang J, Zhang M, Ma QM, Wang XF, Ma ZY (2021). The G-protein α subunit GhGPA positively regulates Gossypium hirsutum resistance to Verticillium dahliae via induction of SA and JA signaling pathways and ROS accumulation. Crop J 9, 823-833. |
| [3] | Chen LB, Ji CH, Zhou DG, Gou X, Tang JN, Jiang YJ, Han JL, Liu YG, Chen LY, Xie YY (2022). OsLTP47 may function in a lipid transfer relay essential for pollen wall development in rice. J Genet Genomics 49, 481-491. |
| [4] | Dai PH, Hu ZY, Li XQ, Lei JF, Liu C, Liu XD, Li Y (2022). Cloning and functional analysis of GhMYB6 gene related to cotton Verticillium wilt resistance. J South Agric 53, 3020-3027. (in Chinese) |
| 代培红, 胡子曜, 李秀青, 雷建峰, 刘超, 刘晓东, 李月 (2022). 棉花黄萎病相关基因GhMYB6的克隆与功能分析. 南方农业学报 53, 3020-3027. | |
| [5] | David L, Kang JN, Nicklay J, Dufresne C, Chen SX (2021). Identification of DIR1-dependant cellular responses in guard cell systemic acquired resistance. Front Mol Biosci 8, 746523. |
| [6] | Dong YM, Zhang WY, Ling ZY, Li JR, Bai HT, Li H, Shi L (2020). Advances in transcription factors regulating plant terpenoids biosynthesis. Chin Bull Bot 55, 340-350. (in Chinese) |
| 董燕梅, 张文颖, 凌正一, 李靖锐, 白红彤, 李慧, 石雷 (2020). 转录因子调控植物萜类化合物生物合成研究进展. 植物学报 55, 340-350. | |
| [7] | Fan YP, Zhang YX, Rui C, Xu N, Zhang H, Wang J, Malik WA, Han MG, Zhao LJ, Lu XK, Chen XG, Chen C, Ye WW (2021). Zinc finger transcription factor ZAT family genes confer multi-tolerances in Gossypium hirsutum L. J Cotton Res 4, 24. |
| [8] | Fradin EF, Thomma BPHJ (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol 7, 71-86. |
| [9] | Gao SQ, Shao WK, Zhao Z, Shao PX, Hu WR, Huang QS (2023). Functional analysis of cotton calcineurin B-like protein GhCBL3-A01 in regulating the resistance to Verticillium wilt. Cotton Sci 35, 447-458. (in Chinese) |
| 高升旗, 邵武奎, 赵准, 邵盘霞, 胡文冉, 黄全生 (2023). 类钙调磷酸酶B亚基蛋白GhCBL3-A01调控棉花黄萎病抗性的功能分析. 棉花学报 35, 447-458. | |
| [10] | Gautam H, Sharma A, Trivedi PK (2023). The role of flavonols in insect resistance and stress response. Curr Opin Plant Biol 73, 102353. |
| [11] | Gfeller A, Dubugnon L, Liechti R, Farmer EE (2010). Jasmonate biochemical pathway. Sci Signal 3, cm3. |
| [12] | Harms K, Atzorn R, Brash A, Kuhn H, Wasternack C, Willmitzer L, Pena-Cortes H (1995). Expression of a flax allene oxide synthase cDNA leads to increased endogenous jasmonic acid (JA) levels in transgenic potato plants but not to a corresponding activation of JA-responding genes. Plant Cell 7, 1645-1654. |
| [13] | Hu XQ, Shi ZY, Zhu YT, Gao LY, Wang P, Wang HW, Hou YX (2023). Mechanism of the cotton GhRAR1 gene regulating the resistance of cotton to Verticillium wilt. Plant Prot 49(2), 39-47, 56. (in Chinese) |
| 胡晓倩, 石志钰, 朱玉涛, 高琳颖, 王平, 王宏伟, 侯玉霞 (2023). 棉花GhRAR1基因调控棉花抗黄萎病机理的研究. 植物保护 49(2), 39-47, 56. | |
| [14] | Hu ZY, Li XQ, Dai PH, Lei JF, Liu JF, Zhao Y, Deng JH, Liu C, Liu XD, Li Y (2022). Functional verification of GhP450-94C1 that a Verticillium wilt resistant gene in Gossypium hirsutum L. Acta Agric Bor-Sin 37(6), 72-81. (in Chinese) |
| 胡子曜, 李秀青, 代培红, 雷建峰, 柳建飞, 赵燚, 邓嘉辉, 刘超, 刘晓东, 李月 (2022). 陆地棉细胞色素P450基因GhP450-94C1黄萎病抗性功能验证. 华北农学报 37(6), 72-81. | |
| [15] | Huang H, Liu B, Liu LY, Song SS (2017). Jasmonate action in plant growth and development. J Exp Bot 68, 1349-1359. |
| [16] | Huang Y, Xie FJ, Cao X, Li MY (2021). Research progress in biosynthesis and regulation of plant terpenoids. Biotechnol Biotechnol Equip 35, 1799-1808. |
| [17] | Huffaker A, Kaplan F, Vaughan MM, Dafoe NJ, Ni XZ, Rocca JR, Alborn HT, Teal PEA, Schmelz EA (2011). Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol 156, 2082-2097. |
| [18] | Jacq A, Pernot C, Martinez Y, Domergue F, Payré B, Jamet E, Burlat V, Pacquit VB (2017). The Arabidopsis lipid transfer protein 2 (AtLTP2) is involved in cuticle-cell wall interface integrity and in etiolated hypocotyl permeability. Front Plant Sci 8, 263. |
| [19] | Kim J, Lee WJ, Vu TT, Jeong CY, Hong SW, Lee H (2017). High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep 36, 1215-1224. |
| [20] | Kumar V, Nadda G, Kumar S, Yadav SK (2013). Transgenic tobacco overexpressing tea cDNA encoding dihydroflavonol 4-reductase and anthocyanidin reductase induces early flowering and provides biotic stress tolerance. PLoS One 8, e65535. |
| [21] | Li MJ (2021). Functional Verification of Cotton GhIQM1, GhNAC90 and GhBsr-d1 Genes in Resistance to Verticillium Wilt. Master’s thesis. Urumqi: Xinjiang Agricultural University. pp. 23-24. (in Chinese) |
| 李名江 (2021). 棉花GhIQM1、GhNAC90和GhBsr-d1基因在抗黄萎病中的功能验证. 硕士论文. 乌鲁木齐: 新疆农业大学. pp. 23-24. | |
| [22] | Liao ZH, Wang L, Li CZ, Cao MJ, Wang JN, Yao ZL, Zhou SY, Zhou GX, Zhang DY, Lou YG (2022). The lipoxygenase gene OsRCI-1 is involved in the biosynthesis of herbivore-induced JAs and regulates plant defense and growth in rice. Plant Cell Environ 45, 2827-2840. |
| [23] | Liu DF, Shi SP, Hao ZJ, Xiong WT, Luo MZ (2019). OsbZIP81, a homologue of Arabidopsis VIP1, may positively regulate JA levels by directly targetting the genes in JA signaling and metabolism pathway in rice. Int J Mol Sci 20, 2360. |
| [24] | Liu PP, von Dahl CC, Park SW, Klessig DF (2011). Interconnection between methyl salicylate and lipid-based long-distance signaling during the development of systemic acquired resistance in Arabidopsis and tobacco. Plant Physiol 155, 1762-1768. |
| [25] | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402-408. |
| [26] | Lu XR, Jia XY, Niu JH (2018). The present situation and prospects of cotton industry development in China. Sci Agric Sin 51, 26-36. (in Chinese) |
| 卢秀茹, 贾肖月, 牛佳慧 (2018). 中国棉花产业发展现状及展望. 中国农业科学 51, 26-36. | |
| [27] | Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK (2002). A putative lipid transfer protein involved in systemic resistance signaling in Arabidopsis. Nature 419, 399-403. |
| [28] | Mansfeld BN, Colle M, Kang YY, Jones AD, Grumet R (2017). Transcriptomic and metabolomic analyses of cucumber fruit peels reveal a developmental increase in terpenoid glycosides associated with age-related resistance to Phytophthora capsici. Hortic Res 4, 17022. |
| [29] | Mo HJ, Wang XF, Zhang Y, Zhang GY, Zhang JF, Ma ZY (2015). Cotton polyamine oxidase is required for spermine and camalexin signaling in the defence response to Verticillium dahliae. Plant J 83, 962-975. |
| [30] | Qanmber G, Lu LL, Liu Z, Yu DQ, Zhou KH, Huo P, Li FG, Yang ZR (2019). Genome-wide identification of GhAAI genes reveals that GhAAI66 triggers a phase transition to induce early flowering. J Exp Bot 70, 4721-4736. |
| [31] | Sanchez S, Demain AL (2008). Metabolic regulation and overproduction of primary metabolites. Microb Biotechnol 1, 283-319. |
| [32] | Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D (2001). Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell 104, 119-130. |
| [33] | Song RR, Li JP, Xie CJ, Jian W, Yang XY (2020). An overview of the molecular genetics of plant resistance to the Verticillium wilt pathogen Verticillium dahliae. Int J Mol Sci 21, 1120. |
| [34] | Su XF, Lu GQ, Guo HM, Zhang KX, Li XK, Cheng HM (2018). The dynamic transcriptome and metabolomics profiling in Verticillium dahliae inoculated Arabidopsis thaliana. Sci Rep 8, 15404. |
| [35] | Thayale Purayil F, Rajashekar B, Kurup SS, Cheruth AJ, Subramaniam S, Hassan Tawfik N, Amiri KMA (2020). Transcriptome profiling of Haloxylon persicum (Bunge ex Boiss and Buhse) an endangered plant species under PEG-induced drought stress. Genes (Basel) 11, 640. |
| [36] | Tholl D (2015). Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol 148, 63-106. |
| [37] | Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI (2022). Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol 23, 680-694. |
| [38] | Wang J, Dong JZ, Chen H, Zheng LS, Wang M (2018). Analysis and forecast of global cotton import and export trade. Cotton Text Technol 46(3), 81-84. (in Chinese) |
| 王健, 董俊哲, 陈浩, 郑丽莎, 王铭 (2018). 全球棉花进出口贸易分析及展望. 棉纺织技术 46(3), 81-84. | |
| [39] | Wang LL, Xu GJ, Li LH, Ruan MY, Bennion A, Wang GL, Li R, Qu SH (2023). The OsBDR1-MPK3 module negatively regulates blast resistance by suppressing the jasmonate signaling and terpenoid biosynthesis pathway. Proc Natl Acad Sci USA 120, e2211102120. |
| [40] | Wang Q, Cao R, Zhang YN, Qi PY, Wang LZ, Fang SM (2021). Biosynthesis and regulation of terpenoids from basidiomycetes: exploration of new research. AMB Express 11, 150. |
| [41] | Wang XF (2007). Studies on Non-Specific Lipid Transfer Protein Receptors on Rice Cell Membranes. PhD dissertation. Shanghai: Fudan University. pp. 64-65. (in Chinese) |
| 汪笑峰 (2007). 水稻细胞膜上非特异性脂质转移蛋白受体的研究. 博士论文. 上海: 复旦大学. pp. 64-65. | |
| [42] | Xie JW, Cao XY, Pan WQ, Du LJ (2024). Advances in plant flavonoid transport and accumulation mechanism. Chin Bull Bot 59, 463-480. (in Chinese) |
| 谢靖雯, 曹晓云, 潘婉琪, 杜灵娟 (2024). 植物类黄酮转运与积累机制的研究进展. 植物学报 59, 463-480. | |
| [43] | Xu YH, Wang JW, Wang S, Wang JY, Chen XY (2004). Characterization of GaWRKY1, a cotton transcription factor that regulates the sesquiterpene synthase gene (+)-δ-cadinene synthase-A. Plant Physiol 135, 507-515. |
| [44] | Yamaguchi Y, Barona G, Ryan CA, Pearce G (2011). GmPep914, an eight-amino acid peptide isolated from soybean leaves, activates defense-related genes. Plant Physiol 156, 932-942. |
| [45] | Yang G, Sun MH, Wang ZF, Hu QY, Guo JJ, Yu J, Lei CZ, Dang RH (2023). Comparative genomics identifies the evolutionarily conserved gene TPM3 as a target of eca-miR-1 involved in the skeletal muscle development of donkeys. Int J Mol Sci 24, 15440. |
| [46] | Yang YX, Ahammed GJ, Wu CJ, Fan SY, Zhou YH (2015). Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr Protein Pept Sci 16, 450-461. |
| [47] | Yu KS, Soares JM, Mandal MK, Wang CX, Chanda B, Gifford AN, Fowler JS, Navarre D, Kachroo A, Kachroo P (2013). A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Rep 3, 1266-1278. |
| [48] | Yu TF, Hou ZH, Wang HL, Chang SY, Song XY, Zheng WJ, Zheng L, Wei JT, Lu ZW, Chen J, Zhou YB, Chen M, Sun SL, Jiang QY, Jin LG, Ma YZ, Xu ZS (2024). Soybean steroids improve crop abiotic stress tolerance and increase yield. Plant Biotechnol J 22, 2333-2347. |
| [49] | Zhang M, Zhang J, Zhang XY, Wang GN, Wang XF, Zhang Y (2023). Cloning and functional analysis of GhNAC1 in upland cotton involved in Verticillium wilt resistance. J Agric Sci Technol 25(10), 35-44. (in Chinese) |
| 张曼, 张进, 张新雨, 王国宁, 王省芬, 张艳 (2023). 陆地棉GhNAC1基因的克隆及抗黄萎病功能分析. 中国农业科技导报 25(10), 35-44. | |
| [50] | Zhu YT, Hu XQ, Wang P, Wang HW, Ge XY, Li FG, Hou YX (2022). GhODO1, an R2R3-type MYB transcription factor, positively regulates cotton resistance to Verticillium dahliae via the lignin biosynthesis and jasmonic acid signaling pathway. Int J Biol Macromol 201, 580-591. |
| [51] | Zulfiqar S, Farooq MA, Zhao TT, Wang PP, Tabusam J, Wang YH, Xuan SX, Zhao JJ, Chen XP, Shen SX, Gu AX (2023). Virus-induced gene silencing (VIGS): a powerful tool for crop improvement and its advancement towards epigenetics. Int J Mol Sci 24, 5608. |
/
| 〈 |
|
〉 |