

植物学报 ›› 2024, Vol. 59 ›› Issue (4): 626-634.DOI: 10.11983/CBB23118 cstr: 32102.14.CBB23118
杨佳丽1,2, 饶羽菲1,2, 张润花3, 周国林3, 林处发3, 何燕红1,2,*( ), 宁国贵1,2,*(
), 宁国贵1,2,*( )
)
收稿日期:2023-08-29
接受日期:2023-11-02
出版日期:2024-07-10
发布日期:2024-07-10
通讯作者:
何燕红,宁国贵
基金资助:
Jiali Yang1,2, Yufei Rao1,2, Runhua Zhang3, Guolin Zhou3, Chufa Lin3, Yanhong He1,2,*( ), Guogui Ning1,2,*(
), Guogui Ning1,2,*( )
)
Received:2023-08-29
Accepted:2023-11-02
Online:2024-07-10
Published:2024-07-10
Contact:
Yanhong He, Guogui Ning
摘要: 以2个不同基因型的捕虫堇圆切捕虫堇(Pinguicula cyclosecta)和塞提捕虫堇(P. ‘Sethos’)叶片为外植体, 通过探究不定芽再生的影响因素, 成功建立了捕虫堇高效再生体系。结果表明, 2种捕虫堇对消毒方式和基本培养基种类要求相同, 但再生能力有显著差异。圆切捕虫堇叶片在MS+1.0 mg∙L-1 6-BA+0.2 mg∙L-1 NAA培养基上再生能力最强, 再生率为92.22%, 再生系数达4.84, 在MS+0.6 mg∙L-1 6-BA+0.1 mg∙L-1 NAA培养基上, 不定芽增殖系数达4.98。塞提捕虫堇叶片在MS+2.0 mg∙L-1 6-BA+0.2 mg∙L-1 NAA培养基中再生率最高, 为77.78%, 再生系数为6.12, 在增殖培养基MS+0.3 mg∙L-1 6-BA+0.1 mg∙L-1 NAA上, 增殖系数可达4.84。2种捕虫堇在1/2MS+0.1 mg∙L-1 IBA培养基上进行生根培养, 根系生长状态最佳。该研究解决了捕虫堇繁殖系数低及工厂化育苗难等问题, 为其规模化生产和育种改良提供了技术支持。
杨佳丽, 饶羽菲, 张润花, 周国林, 林处发, 何燕红, 宁国贵. 捕虫堇叶片高效再生体系建立. 植物学报, 2024, 59(4): 626-634.
Jiali Yang, Yufei Rao, Runhua Zhang, Guolin Zhou, Chufa Lin, Yanhong He, Guogui Ning. Establishment of an Efficient Leaf Regeneration System for Pinguicula. Chinese Bulletin of Botany, 2024, 59(4): 626-634.
| Disinfection scheme | Pollution rate (%) | Survival rate (%) | ||
|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | |
| 0.1% HgCl2 2 min | 40.00±3.33 a | 37.78±5.09 a | 97.78±3.85 a | 89.25±1.29 a |
| 0.1% HgCl2 5 min | 31.11±3.85 b | 18.89±1.92 b | 94.44±5.09 a | 84.44±1.93 b |
| 0.1% HgCl2 8 min | 13.33±3.34 d | 5.56±1.93 d | 81.11±1.92 bc | 74.44±1.93 c |
| 75% alcohol 10 s+0.1% HgCl2 2 min | 17.78±1.92 c | 12.22±1.92 c | 95.55±3.85 a | 81.11±1.92 b |
| 75% alcohol 10 s+0.1% HgCl2 5 min | 7.78±1.92 e | 4.44±1.93 de | 86.67±3.34 b | 71.11±1.92 c |
| 75% alcohol 10 s+0.1% HgCl2 8 min | 1.11±1.92 f | 0.00±0.00 f | 75.56±1.93 cd | 64.45±3.85 d |
| 75% alcohol 20 s+0.1% HgCl2 2 min | 4.44±1.93 ef | 1.11±1.92 ef | 74.45±3.85 d | 66.67±3.34 d |
| 75% alcohol 20 s+0.1% HgCl2 5 min | 0.00±0.00 f | 0.00±0.00 f | 64.78±2.51 e | 57.78±1.92 e |
| 75% alcohol 20 s+0.1% HgCl2 8 min | 0.00±0.00 f | 0.00±0.00 f | 61.11±1.92 e | 47.78±1.92 f |
表1 不同消毒方式对2种捕虫堇污染率和存活率的影响
Table 1 Effect of disinfection scheme on pollution rate and survival rate of two Pinguicula species
| Disinfection scheme | Pollution rate (%) | Survival rate (%) | ||
|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | |
| 0.1% HgCl2 2 min | 40.00±3.33 a | 37.78±5.09 a | 97.78±3.85 a | 89.25±1.29 a |
| 0.1% HgCl2 5 min | 31.11±3.85 b | 18.89±1.92 b | 94.44±5.09 a | 84.44±1.93 b |
| 0.1% HgCl2 8 min | 13.33±3.34 d | 5.56±1.93 d | 81.11±1.92 bc | 74.44±1.93 c |
| 75% alcohol 10 s+0.1% HgCl2 2 min | 17.78±1.92 c | 12.22±1.92 c | 95.55±3.85 a | 81.11±1.92 b |
| 75% alcohol 10 s+0.1% HgCl2 5 min | 7.78±1.92 e | 4.44±1.93 de | 86.67±3.34 b | 71.11±1.92 c |
| 75% alcohol 10 s+0.1% HgCl2 8 min | 1.11±1.92 f | 0.00±0.00 f | 75.56±1.93 cd | 64.45±3.85 d |
| 75% alcohol 20 s+0.1% HgCl2 2 min | 4.44±1.93 ef | 1.11±1.92 ef | 74.45±3.85 d | 66.67±3.34 d |
| 75% alcohol 20 s+0.1% HgCl2 5 min | 0.00±0.00 f | 0.00±0.00 f | 64.78±2.51 e | 57.78±1.92 e |
| 75% alcohol 20 s+0.1% HgCl2 8 min | 0.00±0.00 f | 0.00±0.00 f | 61.11±1.92 e | 47.78±1.92 f |
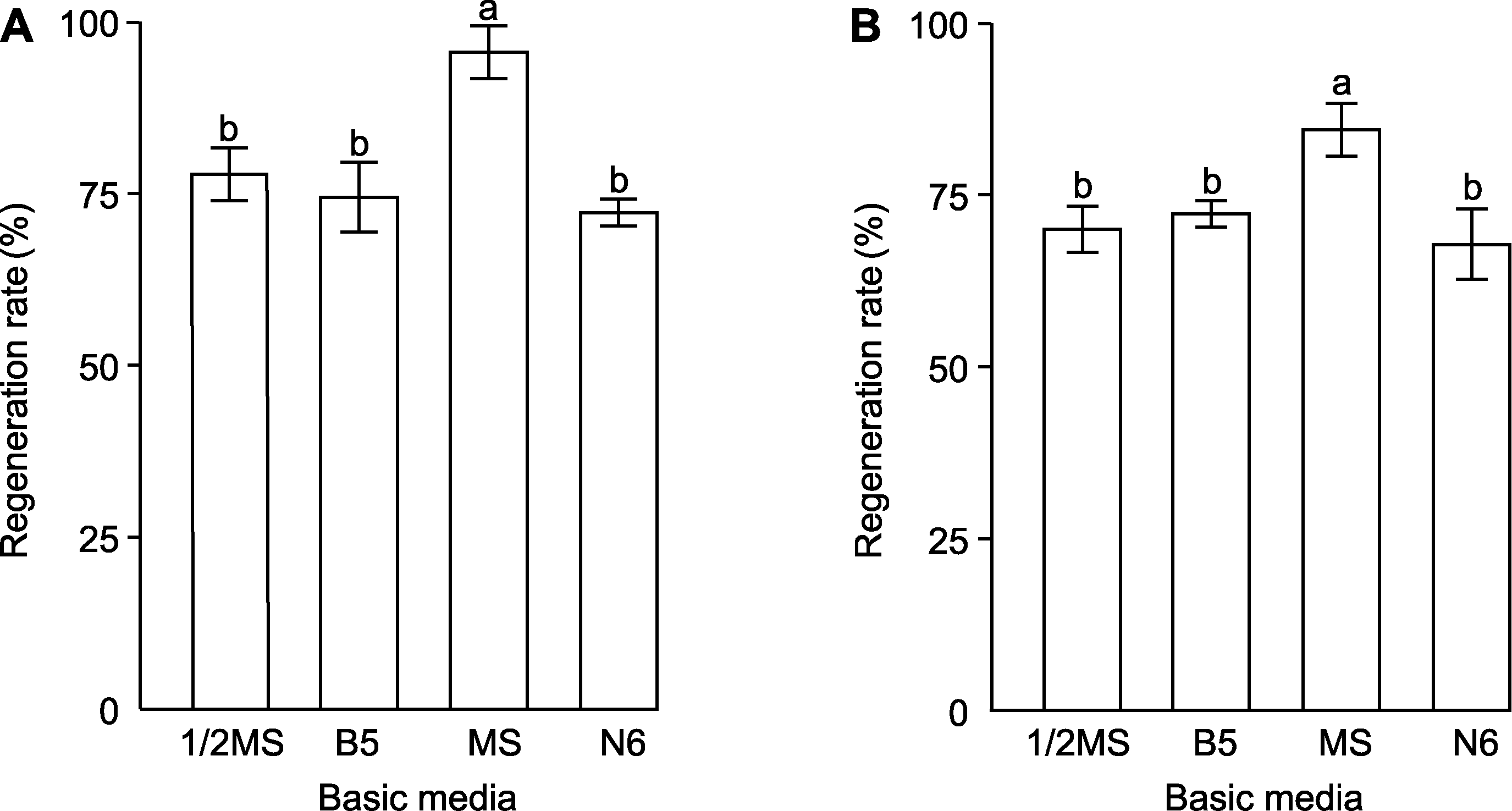
图1 不同基本培养基对圆切捕虫堇和塞提捕虫堇叶片再生的影响 (A) 圆切捕虫堇再生率; (B) 塞提捕虫堇再生率。不同小写字母表示各处理间差异显著(P<0.05)。
Figure 1 Effects of different basic media on the leaf regeneration of Pinguicula cyclosecta and P. ‘Sethos’ (A) Regeneration rate of P. cyclosecta; (B) Regeneration rate of P. ‘Sethos’. Different lowercase letters indicate significant differences among different treatments (P<0.05).
| 6-BA (mg·L-1) | NAA (mg·L-1) | Regeneration rate (%) | Regeneration coefficient | |||
|---|---|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | |||
| 0.5 | 0 | 87.78±3.85 bc | 72.22±5.09 c | 3.60±0.98 cd | 3.52±0.99 d | |
| 0.5 | 0.2 | 92.22±3.85 b | 81.11±1.93 b | 3.96±1.28 bc | 4.14±1.03 bc | |
| 0.5 | 0.5 | 83.33±3.33 c | 75.56±3.85 bc | 3.61±0.82 cd | 4.02±0.96 c | |
| 1.0 | 0 | 98.89±1.93 a | 91.11±1.93 a | 3.60±0.96 cd | 3.94±0.87 c | |
| 1.0 | 0.2 | 92.22±1.93 b | 91.11±3.85 a | 4.84±0.95 a | 4.04±0.96 c | |
| 1.0 | 0.5 | 82.22±1.93 c | 77.78±1.93 bc | 4.32±0.86 b | 3.84±0.79 cd | |
| 2.0 | 0 | 85.56±1.93 c | 76.67±3.33 ab | 4.04±0.85 b | 4.38±0.81 b | |
| 2.0 | 0.2 | 83.33±6.67 c | 77.78±3.85 bc | 3.36±0.79 d | 6.12±0.97 a | |
| 2.0 | 0.5 | 84.44±5.09 c | 72.22±3.85 c | 2.54±0.88 ef | 5.96±0.82 a | |
| 3.0 | 0 | 84.44±3.85 c | 71.11±5.09 c | 2.82±0.95 e | 1.90±0.71 e | |
| 3.0 | 0.2 | 82.22±1.93 c | 75.56±5.09 bc | 2.24±0.70 fg | 2.08±0.60 e | |
| 3.0 | 0.5 | 72.22±1.93 d | 72.22±3.85 c | 2.02±0.68 g | 2.11±0.72 e | |
表2 植物生长调节剂对2种捕虫堇叶片再生的影响
Table 2 Effect of plant growth regulators on leaf regeneration of two Pinguicula species
| 6-BA (mg·L-1) | NAA (mg·L-1) | Regeneration rate (%) | Regeneration coefficient | |||
|---|---|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | |||
| 0.5 | 0 | 87.78±3.85 bc | 72.22±5.09 c | 3.60±0.98 cd | 3.52±0.99 d | |
| 0.5 | 0.2 | 92.22±3.85 b | 81.11±1.93 b | 3.96±1.28 bc | 4.14±1.03 bc | |
| 0.5 | 0.5 | 83.33±3.33 c | 75.56±3.85 bc | 3.61±0.82 cd | 4.02±0.96 c | |
| 1.0 | 0 | 98.89±1.93 a | 91.11±1.93 a | 3.60±0.96 cd | 3.94±0.87 c | |
| 1.0 | 0.2 | 92.22±1.93 b | 91.11±3.85 a | 4.84±0.95 a | 4.04±0.96 c | |
| 1.0 | 0.5 | 82.22±1.93 c | 77.78±1.93 bc | 4.32±0.86 b | 3.84±0.79 cd | |
| 2.0 | 0 | 85.56±1.93 c | 76.67±3.33 ab | 4.04±0.85 b | 4.38±0.81 b | |
| 2.0 | 0.2 | 83.33±6.67 c | 77.78±3.85 bc | 3.36±0.79 d | 6.12±0.97 a | |
| 2.0 | 0.5 | 84.44±5.09 c | 72.22±3.85 c | 2.54±0.88 ef | 5.96±0.82 a | |
| 3.0 | 0 | 84.44±3.85 c | 71.11±5.09 c | 2.82±0.95 e | 1.90±0.71 e | |
| 3.0 | 0.2 | 82.22±1.93 c | 75.56±5.09 bc | 2.24±0.70 fg | 2.08±0.60 e | |
| 3.0 | 0.5 | 72.22±1.93 d | 72.22±3.85 c | 2.02±0.68 g | 2.11±0.72 e | |
| 6-BA (mg·L-1) | NAA (mg·L-1) | Proliferation coefficient | Growth situation | ||
|---|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | ||
| 0.3 | 0.05 | 3.24±0.87 d | 2.66±0.87 c | + | ++ |
| 0.3 | 0.1 | 3.50±0.95 d | 4.84±0.93 a | ++ | ++++ |
| 0.6 | 0.05 | 4.36±0.92 b | 3.52±0.85 b | +++ | + |
| 0.6 | 0.1 | 4.98±0.94 a | 3.96±0.84 b | ++++ | ++ |
| 1.0 | 0.05 | 4.04±1.03 c | 2.90±0.93 bc | ++ | ++ |
| 1.0 | 0.1 | 4.18±0.77 bc | 2.80±0.85 bc | ++ | ++ |
表3 植物生长调节剂对2种捕虫堇不定芽增殖的影响
Table 3 Effects of plant growth regulators on the adventitious bud proliferation of two Pinguicula species
| 6-BA (mg·L-1) | NAA (mg·L-1) | Proliferation coefficient | Growth situation | ||
|---|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | ||
| 0.3 | 0.05 | 3.24±0.87 d | 2.66±0.87 c | + | ++ |
| 0.3 | 0.1 | 3.50±0.95 d | 4.84±0.93 a | ++ | ++++ |
| 0.6 | 0.05 | 4.36±0.92 b | 3.52±0.85 b | +++ | + |
| 0.6 | 0.1 | 4.98±0.94 a | 3.96±0.84 b | ++++ | ++ |
| 1.0 | 0.05 | 4.04±1.03 c | 2.90±0.93 bc | ++ | ++ |
| 1.0 | 0.1 | 4.18±0.77 bc | 2.80±0.85 bc | ++ | ++ |
| No. | NAA (mg·L-1) | IBA (mg·L-1) | Average rooting number | Root length (cm) | ||
|---|---|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | |||
| 1 | 0.1 | 0 | 7.13±1.33 d | 9.95±1.08 d | 0.89±0.96 d | 3.97±0.80 c |
| 2 | 0.2 | 0 | 6.97±1.11 d | 12.03±1.70 b | 0.76±1.40 e | 4.86±0.84 b |
| 3 | 0.3 | 0 | 5.30±0.74 e | 9.90±0.90 d | 0.63±0.74 f | 3.02±0.86 d |
| 4 | 0 | 0.1 | 11.00±1.15 a | 13.25±1.28 a | 1.69±0.75 a | 5.58±0.68 a |
| 5 | 0 | 0.2 | 9.70±0.78 b | 11.73±0.93 b | 1.55±0.84 b | 4.23±1.03 c |
| 6 | 0 | 0.3 | 8.07±0.89 c | 10.88±1.26 c | 1.31±0.81 c | 4.08±0.89 c |
表4 植物生长调节剂对2种捕虫堇不定芽生根的影响
Table 4 Effects of plant growth regulators on the adventitious bud rooting of two Pinguicula species
| No. | NAA (mg·L-1) | IBA (mg·L-1) | Average rooting number | Root length (cm) | ||
|---|---|---|---|---|---|---|
| P. cyclosecta | P. ‘Sethos’ | P. cyclosecta | P. ‘Sethos’ | |||
| 1 | 0.1 | 0 | 7.13±1.33 d | 9.95±1.08 d | 0.89±0.96 d | 3.97±0.80 c |
| 2 | 0.2 | 0 | 6.97±1.11 d | 12.03±1.70 b | 0.76±1.40 e | 4.86±0.84 b |
| 3 | 0.3 | 0 | 5.30±0.74 e | 9.90±0.90 d | 0.63±0.74 f | 3.02±0.86 d |
| 4 | 0 | 0.1 | 11.00±1.15 a | 13.25±1.28 a | 1.69±0.75 a | 5.58±0.68 a |
| 5 | 0 | 0.2 | 9.70±0.78 b | 11.73±0.93 b | 1.55±0.84 b | 4.23±1.03 c |
| 6 | 0 | 0.3 | 8.07±0.89 c | 10.88±1.26 c | 1.31±0.81 c | 4.08±0.89 c |

图2 圆切捕虫堇叶片再生过程 (A) 圆切捕虫堇; (B) 外植体; (C), (D) 叶片在MS+1.0 mg∙L-1 6-BA+0.2 mg∙L-1 NAA培养基上培养30天; (E), (F) 不定芽在MS+ 0.6 mg∙L-1 6-BA+0.1 mg∙L-1 NAA培养基上增殖培养30天; (G), (H) 增殖芽在1/2MS+0.1 mg∙L-1 IBA培养基上生根培养30天; (I) 移栽30天后成活的再生植株。Bars=1 cm
Figure 2 Leaf regeneration process of Pinguicula cyclosecta (A) P. cyclosecta; (B) Explants; (C), (D) Leaves regenerated on MS+1.0 mg∙L-1 6-BA+0.2 mg∙L-1 NAA media for 30 days; (E), (F) Buds grew on MS+0.6 mg∙L-1 6-BA+0.1 mg∙L-1 NAA media for 30 days; (G), (H) The fascicular buds rooted on 1/2MS+ 0.1 mg∙L-1 IBA media for 30 days; (I) Regenerated plants that are viable after 30 days of transplanting. Bars=1 cm

图3 塞提捕虫堇叶片再生过程 (A) 塞提捕虫堇; (B) 外植体; (C), (D) 叶片在MS+2.0 mg∙L-1 6-BA+0.2 mg∙L-1 NAA培养基上培养30天; (E), (F) 不定芽在MS+ 0.3 mg∙L-1 6-BA+0.1 mg∙L-1 NAA培养基上增殖培养30天; (G), (H) 增殖芽在1/2MS+0.1 mg∙L-1 IBA培养基上生根培养30天; (I) 移栽30天后成活的再生植株。Bars=1 cm
Figure 3 Leaf regeneration process of Pinguicula ‘Sethos’ (A) P. ‘Sethos’; (B) Explants; (C), (D) Leaves regenerated on MS+2.0 mg∙L-1 6-BA+0.2 mg∙L-1 NAA media for 30 days; (E), (F) Buds grew on MS+0.3 mg∙L-1 6-BA+0.1 mg∙L-1 NAA media for 30 days; (G), (H) The fascicular buds rooted on 1/2MS+ 0.1 mg∙L-1 IBA media for 30 days; (I) Regenerated plants that are viable after 30 days of transplanting. Bars=1 cm
| [1] | 陈英转, 孟新亚, 宋希强, 田代科, 周韬 (2023). 盾叶秋海棠叶片高频再生体系的建立. 植物生理学报 59, 165-171. |
| [2] | 李春华, 李柯澄 (2020). 捕虫堇温室生产与繁殖. 中国花卉园艺 (14), 24-28. |
| [3] | 梁展钊, 梁达新, 赖一航, 叶燕 (2020). 一种捕虫堇组培快繁的方法. 中国专利, CN109220802B. 2020-02-14. |
| [4] |
廖敏凌, 蒲娅, 武晓云, 马朝峰, 王文奎, 戴思兰 (2023). 平潭野菊混合瓣型株系再生体系的建立. 植物学报 58, 449- 460.
DOI |
| [5] |
逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧 (2022). 大花银莲花愈伤组织诱导及再生体系的建立. 植物学报 57, 217-226.
DOI |
| [6] | 彭爱红, 何永睿, 邹修平, 许兰珍, 邹哨兵 (2002). 观赏植物组织培养与基因工程研究进展(综述). 亚热带植物科学 31(2), 58-63. |
| [7] | 曲晓慧 (2020). 几种菊属野生植物再生体系的建立. 硕士论文. 南京: 南京农业大学. pp. 1-43. |
| [8] | 任毅, 刘明时, 田联会, 田先华, 李智军 (2006). 太白山自然保护区生物多样性研究与管理. 北京: 中国林业出版社. pp. 256. |
| [9] | 宋倩, 卢家仕, 周锦业, 周主贵, 李春牛, 卜朝阳 (2015). 食虫植物保护现状及其研究进展. 北方园艺 (3), 171-175. |
| [10] |
王春夏, 张梦迪, 王锦霞, 王志平, 孙红梅 (2020). 朱顶红无菌苗叶片高效再生体系. 园艺学报 47, 301-309.
DOI |
| [11] |
王江英, 汤雪燕, 葛金涛, 邵小斌, 孙明伟 (2023). 四季茶花夏咏国色叶片愈伤诱导与植株再生研究. 浙江农业科学 64, 390-395.
DOI |
| [12] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749- 759. |
| [13] | 吴之坤, 张长芹, 程治英 (2005). 不同培养基质及条件对三种报春花种子萌发及幼苗生长的影响. 种子 24(4), 1-5. |
| [14] | 咸宏康, 支秋娟, 李卉, 王长泉 (2019). 金樱子(Rosa laevigata Michx.)叶片直接再生不定芽体系的建立. 北方园艺 (13), 93-100. |
| [15] | 张清凤, 赵洁, 普春, 李啟菊, 马梅见 (2022). 蝴蝶兰组织培养再生体系的建立. 现代农业科技 (22), 116-118. |
| [16] | 张彦文, 王海洋 (2000). 食虫植物研究的现状和趋势. 广西植物 20, 88-93. |
| [17] | Corredoira E, Costa RL (2021). Application of tissue culture in plant reproduction. Forests 12, 342. |
| [18] | Grogg D, Rohner M, Yates S, Manzanares C, Bull SE, Dalton S, Bosch M, Studer B, Broggini GAL (2022). Callus induction from diverse explants and genotypes enables robust transformation of perennial ryegrass (Lolium perenne L.). Plants 11, 2054. |
| [19] | Ikeuchi M, Sugimoto K, Iwase A (2013). Plant callus: mechanisms of induction and repression. Plant Cell 25, 3159-3173. |
| [20] |
Karlsson PS, Nordell KO, Eirefelt S, Svensson A (1987). Trapping efficiency of three carnivorous Pinguicula species. Oecologia 73, 518-521.
DOI PMID |
| [21] | Krowiak A, Herren CM, Webert KC, Einarsson Á, Hoekman D, Jackson RD, Ives AR (2017). Resource gradients and the distribution and flowering of butterwort, a carnivorous plant. Ann Zool Fenn 54, 163-173. |
| [22] | Mehbub H, Akter A, Akter MA, Mandal MSH, Hoque MA, Tuleja M, Mehraj H (2022). Tissue culture in ornamentals: cultivation factors, propagation techniques, and its application. Plants 11, 3208. |
| [23] | Phillips GC, Garda M (2019). Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol-Plant 55, 242-257. |
| [24] | Villegas SG, Alcalá RE (2018). Reproductive ecology of the carnivorous plant Pinguicula moranensis (Lentibulariaceae). Plant Biol 20, 205-212. |
| [25] | Wójciak M, Feldo M, Stolarczyk P, Płachno BJ (2023). Carnivorous plants from Nepenthaceae and Droseraceae as a source of secondary metabolites. Molecules 28, 2155. |
| [26] |
Zhai N, Xu L (2021). Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat Plants 7, 1453-1460.
DOI PMID |
| [1] | 任露露, 张有泽, 黄克林, 宛晓春, 张照亮, 朱木兰, 韦朝领. 茶树茎段不定芽高效发生体系的建立[J]. 植物学报, 2023, 58(2): 308-315. |
| [2] | 王燕, 牟豪杰, 吕永平, 李海营, 汪一婷, 陈剑平. 寿锦的离体植株再生及组培产业化增殖[J]. 植物学报, 2017, 52(3): 331-336. |
| [3] | 李志军, 焦培培, 周正立, 李倩, 李健强. 灰叶胡杨根蘖繁殖的形态解剖学特征[J]. 植物学报, 2012, 47(2): 133-140. |
| [4] | 张志扬 陈信波 张瑜 龙松华 高原 刘爱玲. 亚麻组织培养高频不定芽诱导体系[J]. 植物学报, 2007, 24(05): 629-635. |
| [5] | 赵术珍 阮圆 王宝山. 盐地碱蓬幼嫩花序的组织培养及植株再生[J]. 植物学报, 2006, 23(1): 52-55. |
| [6] | 徐品三 栾雨时 刘纪文 新美芳二. 百合不定芽培养脱毒种球生产的研究[J]. 植物学报, 2003, 20(03): 313-318. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||