

植物学报 ›› 2024, Vol. 59 ›› Issue (1): 134-143.DOI: 10.11983/CBB22232 cstr: 32102.14.CBB22232
收稿日期:2022-10-03
接受日期:2023-04-18
出版日期:2024-01-10
发布日期:2024-01-10
通讯作者:
*E-mail: 基金资助:
Lu Zhu, Chong Yuan, Yifei Liu*( )
)
Received:2022-10-03
Accepted:2023-04-18
Online:2024-01-10
Published:2024-01-10
Contact:
*E-mail: 摘要: 植物产生的次生代谢产物为人类提供了丰富的药物、香料和工业原料。随着分子生物学和基因组学研究的快速发展, 目前已解析了多种植物的次生代谢产物生物合成基因簇(BGCs)。这为我们快速获取目标产物的生物合成通路和发掘新颖的天然产物开辟了新路径。该文重点围绕植物次生代谢产物生物合成基因簇的定义和特点、基本结构模型与演化以及调控机制等进行综述, 以期为相关研究提供理论依据和借鉴。
朱璐, 袁冲, 刘义飞. 植物次生代谢产物生物合成基因簇研究进展. 植物学报, 2024, 59(1): 134-143.
Lu Zhu, Chong Yuan, Yifei Liu. Research Progress on Plant Secondary Metabolite Biosyn-thetic Gene Clusters. Chinese Bulletin of Botany, 2024, 59(1): 134-143.
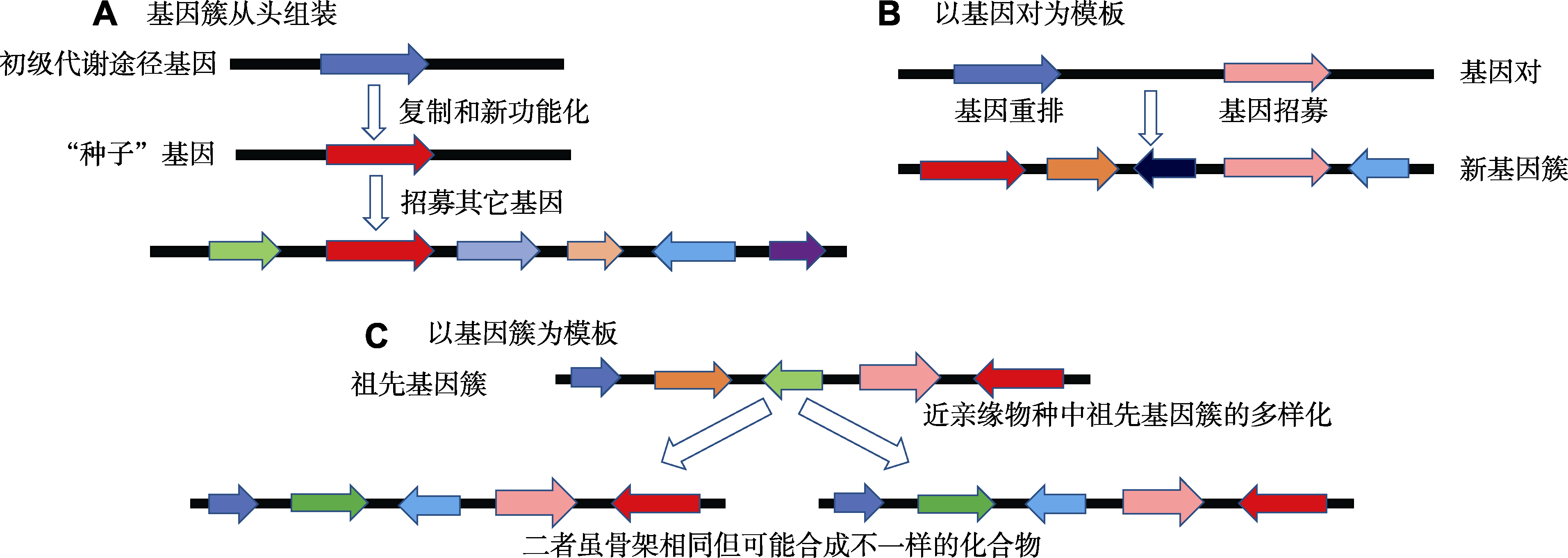
图2 植物生物合成基因簇起源的3种可能方式(改自Nützmann et al., 2016)
Figure 2 Three possible scenarios for the origin of biosynthetic gene clusters in plants (modified from Nützmann et al., 2016)
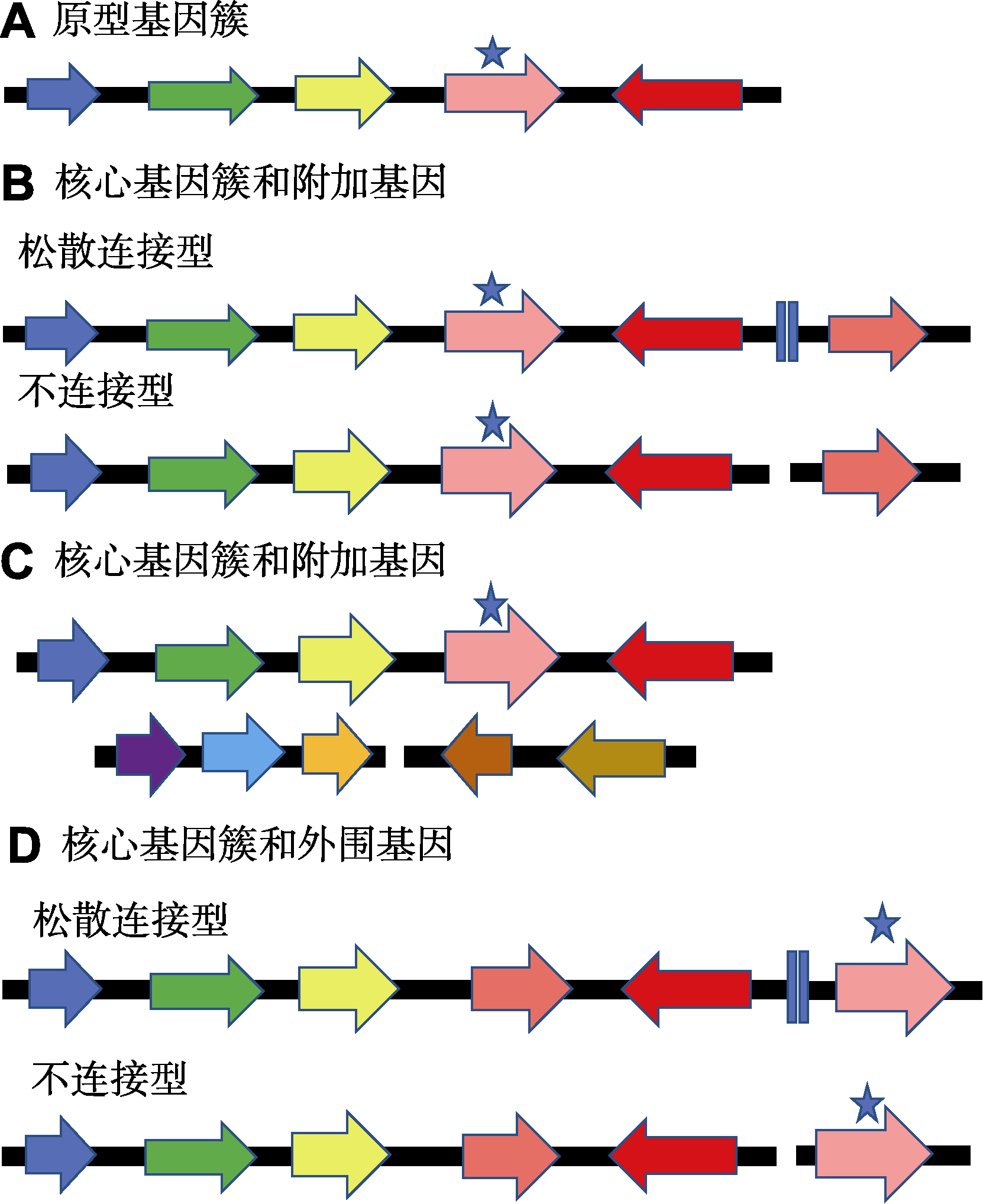
图3 植物次生代谢物生物合成基因簇的4种结构模型(改自Nützmann et al., 2016) *信号基因
Figure 3 Four basic structural models of plant secondary metabolite biosynthesis gene clusters (modified from Nützmann et al., 2016) *Signature genes
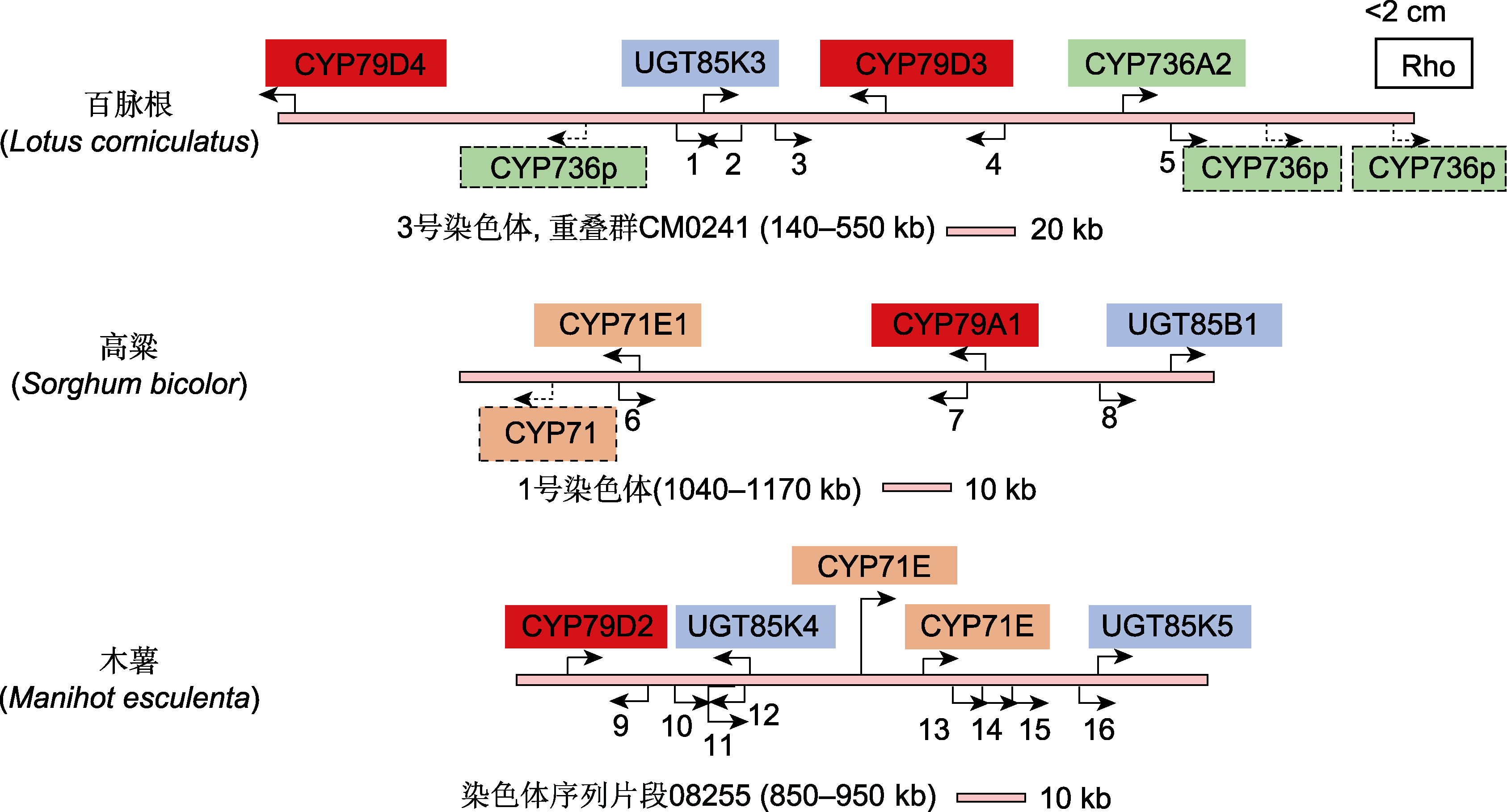
图4 3种典型植物中生氰糖苷生物合成基因簇(改自Takos et al., 2011) 图中箭头代表基因的方向, 方框表示已确认的生氰糖苷生物合成基因, 其中CYP79基因标记为红色方框, CYP71和CYP736基因分别标记为橙色和绿色方框, UGT85基因为蓝色方框。百脉根染色体下方为与CYP736A2相似的3个假基因, 高粱染色体下方显示的为假基因CYP71。Rho基因座位于CM0241等位基因的2 cM范围内。其余基因的编号和注释如下: (1) 顺式还原酮加双氧酶; (2) 核酸结合, OB折叠; (3), (4) 假设蛋白; (5) 核糖核酸酶H; (6) 短链脱氢/还原酶; (7), (8) 假定转座酶; (9) 富亮氨酸重复受体样蛋白激酶; (10), (11) α/β-折叠水解酶; (12) 假设蛋白; (13)-(15) 假定羟腈裂解酶; (16) 假设蛋白。
Figure 4 Genomic clustering of cyanogenic glucoside biosynthetic genes in three typical plants (modified from Takos et al., 2011) Functional genes are presented by arrows indicating their orientation. Confirmed genes in cyanogenic glycoside biosynthesis are labelled above each bar, with CYP79 genes in red, CYP71 genes in orange, CYP736 genes in green, and UGT85 genes in blue. The three CYP736A2-like pseudo-genes are indicated below the Lotus corniculatus bar, as is the additional CYP71 in Sorghum bicolor. The Rho locus is within 2 cM of the CM0241 contig. The remaining genes are numbered and annotated as follows: (1) Acireductone dioxygenase; (2) Nucleic acid binding, OB-fold; (3), (4) Hypothetical proteins; (5) Ribonuclease H; (6) Short-chain dehydrogenase/reductase; (7), (8) Putative transposases; (9) Leucine-rich repeat receptor-like protein kinase; (10), (11) α/β-fold hydrolases; (12) Hypothetical protein; (13)-(15) Putative hydroxynitrile lyases; (16) Hypothetical protein.
| [1] |
方荣俊, 赵华, 廖永辉, 汤程贻, 吴凤瑶, 朱煜, 庞延军, 陆桂华, 王小明, 杨荣武, 戚金亮, 杨永华 (2014). 乙烯对植物次生代谢产物合成的双重调控效应. 植物学报 49, 626-639.
DOI |
| [2] | 吕海舟, 刘琬菁, 何柳, 徐志超, 罗红梅 (2017). 植物次生代谢基因簇研究进展. 植物科学学报 35, 609-621. |
| [3] |
杨谦, 程伯涛, 汤志军, 刘文 (2021). 基因组挖掘在天然产物发现中的应用和前景. 合成生物学 2, 697-715.
DOI |
| [4] |
Bharadwaj R, Kumar SR, Sharma A, Sathishkumar R (2021). Plant metabolic gene clusters: evolution, organization, and their applications in synthetic biology. Front Plant Sci 12, 697318.
DOI URL |
| [5] |
Biere A, Marak HB, van Damme JMM (2004). Plant chemical defense against herbivores and pathogens: generalized defense or trade-offs? Oecologia 140, 430-441.
PMID |
| [6] |
Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T (2019). antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47, W81-W87.
DOI |
| [7] | Boutanaev AM, Moses T, Zi JC, Nelson DR, Mugford ST, Peters RJ, Osbourn A (2015). Investigation of terpene diversification across multiple sequenced plant genomes. Proc Natl Acad Sci USA 112, E81-E88. |
| [8] |
Chomicki G, Schaefer H, Renner SS (2020). Origin and domestication of Cucurbitaceae crops: insights from phylogenies, genomics and archaeology. New Phytol 226, 1240-1255.
DOI PMID |
| [9] |
Darbani B, Motawia MS, Olsen CE, Nour-Eldin HH, Møller BL, Rook F (2016). The biosynthetic gene cluster for the cyanogenic glucoside dhurrin in Sorghum bicolor contains its co-expressed vacuolar MATE transporter. Sci Rep 6, 37079.
DOI |
| [10] |
Field B, Fiston-Lavier AS, Kemen A, Geisler K, Quesne-ville H, Osbourn AE (2011). Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proc Natl Acad Sci USA 108, 16116-16121.
DOI PMID |
| [11] |
Field B, Osbourn AE (2008). Metabolic diversification-independent assembly of operon-like gene clusters in different plants. Science 320, 543-547.
DOI PMID |
| [12] |
Frey M, Chomet P, Glawischnig E, Stettner C, Grun S, Winklmair A, Eisenreich W, Bacher A, Meeley RB, Briggs SP, Simcox K, Gierl A (1997). Analysis of a chemical plant defense mechanism in grasses. Science 277, 696-699.
DOI PMID |
| [13] |
Gaquerel E, Gulati J, Baldwin IT (2014). Revealing insect herbivory-induced phenolamide metabolism: from single genes to metabolic network plasticity analysis. Plant J 79, 679-692.
DOI URL |
| [14] |
Ghanbarian AT, Hurst LD (2015). Neighboring genes show correlated evolution in gene expression. Mol Biol Evol 32, 1748-1766.
DOI PMID |
| [15] |
Guo L, Winzer T, Yang XF, Li Y, Ning ZM, He ZS, Teodor R, Lu Y, Bowser TA, Graham IA, Ye K (2018). The opium poppy genome and morphinan production. Science 362, 343-347.
DOI PMID |
| [16] |
Guo LB, Qiu J, Ye CY, Jin GL, Mao LF, Zhang HQ, Yang XF, Peng Q, Wang YY, Jia L, Lin ZX, Li GM, Fu F, Liu C, Chen L, Shen EH, Wang WD, Chu QJ, Wu DY, Wu SL, Xia CY, Zhang YF, Zhou XM, Wang LF, Wu LM, Song WJ, Wang YF, Shu QY, Aoki D, Yumoto E, Yokota T, Miyamoto K, Okada K, Kim DS, Cai DG, Zhang CL, Lou YG, Qian Q, Yamaguchi H, Yamane H, Kong CH, Timko MP, Bai LY, Fan LJ (2017). Echinochloa crusgalli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat Commun 8, 1031.
DOI |
| [17] |
Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn A (2001). A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc Natl Acad Sci USA 98, 13431-13436.
PMID |
| [18] |
Hen-Avivi S, Savin O, Racovita RC, Lee WS, Adamski NM, Malitsky S, Almekias-Siegl E, Levy M, Vautrin S, Bergès H, Friedlander G, Kartvelishvily E, Ben-Zvi G, Alkan N, Uauy C, Kanyuka K, Jetter R, Distelfeld A, Aharoni A (2016). A metabolic gene cluster in the wheat W1 and the barley Cer-cqu loci determines β-diketone biosynthesis and glaucousness. Plant Cell 28, 1440-1460.
DOI URL |
| [19] |
Itkin M, Heinig U, Tzfadia O, Bhide AJ, Shinde B, Cardenas PD, Bocobza SE, Unger T, Malitsky S, Finkers R, Tikunov Y, Bovy A, Chikate Y, Singh P, Rogachev I, Beekwilder J, Giri AP, Aharoni A (2013). Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 341, 175-179.
DOI PMID |
| [20] |
Jeon JE, Kim JG, Fischer CR, Mehta N, Dufour-Schroif C, Wemmer K, Mudgett MB, Sattely E (2020). A pathogen-responsive gene cluster for highly modified fatty acids in tomato. Cell 180, 176-187.
DOI PMID |
| [21] |
Jonczyk R, Schmidt H, Osterrieder A, Fiesselmann A, Schullehner K, Haslbeck M, Sicker D, Hofmann D, Yalpani N, Simmons C, Frey M, Gierl A (2008). Elucidation of the final reactions of DIMBOA-glucoside biosynthesis in maize: characterization of Bx6 and Bx7. Plant Physiol 146, 1053-1063.
DOI PMID |
| [22] |
Jones DA (1998). Why are so many food plants cyanogenic? Phytochemistry 47, 155-162.
PMID |
| [23] |
Kakes P (1989). An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theor Appl Genet 77, 111-118.
DOI PMID |
| [24] |
Kariya K, Ube N, Ueno M, Teraishi M, Okumoto Y, Mori N, Ueno K, Ishihara A (2020). Natural variation of diterpenoid phytoalexins in cultivated and wild rice species. Phytochemistry 180, 112518.
DOI PMID |
| [25] |
Komaki H, Sakurai K, Hosoyama A, Kimura A, Igarashi Y, Tamura T (2018). Diversity of nonribosomal peptide synthetase and polyketide synthase gene clusters among taxonomically close Streptomyces strains. Sci Rep 8, 6888.
DOI |
| [26] |
Li DD, Bi XY, Ma JJ, Zhang XH, Jiang KN, Zhu XZ, Huang JG, Zhou LJ (2022). Natural herbicidal alkaloid berberine regulates the expression of thalianol and marneral gene clusters in Arabidopsis thaliana. Pest Manag Sci 78, 2896-2908.
DOI URL |
| [27] |
Li DP, Gaquerel E (2021). Next-generation mass spectrometry metabolomics revives the functional analysis of plant metabolic diversity. Annu Rev Plant Biol 72, 867-891.
DOI URL |
| [28] |
Liu ZH, Suarez Duran HG, Harnvanichvech Y, Stephenson MJ, Schranz ME, Nelson D, Medema MH, Osbourn A (2020). Drivers of metabolic diversification: how dynamic genomic neighbourhoods generate new biosynthetic pathways in the Brassicaceae. New Phytol 227, 1109-1123.
DOI PMID |
| [29] |
Luo C, Fernie AR, Yan JB (2020). Single-cell genomics and epigenomics: technologies and applications in plants. Trends Plant Sci 25, 1030-1040.
DOI PMID |
| [30] |
Matsuba Y, Nguyen TTH, Wiegert K, Falara V, Gonzales-Vigil E, Leong B, Schäfer P, Kudrna D, Wing RA, Bolger AM, Usadel B, Tissier A, Fernie AR, Barry CS, Pichersky E (2013). Evolution of a complex locus for terpene biosynthesis in Solanum. Plant Cell 25, 2022-2036.
DOI URL |
| [31] |
Nelson D, Werck-Reichhart D (2011). A P450-centric view of plant evolution. Plant J 66, 194-211.
DOI URL |
| [32] |
Netzker T, Fischer J, Weber J, Mattern DJ, König CC, Valiante V, Schroeckh V, Brakhage AA (2015). Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front Microbiol 6, 299.
DOI PMID |
| [33] |
Nützmann HW, Doerr D, Ramírez-Colmenero A, Sotelo- Fonseca JE, Wegel E, Di Stefano M, Wingett SW, Fraser P, Hurst L, Fernandez-Valverde SL, Osbourn A (2020). Active and repressed biosynthetic gene clusters have spatially distinct chromosome states. Proc Natl Acad Sci USA 117, 13800-13809.
DOI URL |
| [34] |
Nützmann HW, Huang AC, Osbourn A (2016). Plant metabolic clusters—from genetics to genomics. New Phytol 211, 771-789.
DOI URL |
| [35] |
Nützmann HW, Osbourn A (2014). Gene clustering in plant specialized metabolism. Curr Opin Biotechnol 26, 91-99.
DOI URL |
| [36] |
Nützmann HW, Scazzocchio C, Osbourn A (2018). Metabolic gene clusters in eukaryotes. Annu Rev Genet 52, 159-183.
DOI URL |
| [37] |
Okada BK, Wu YH, Mao DN, Bushin LB, Seyedsayam-dost MR (2016). Mapping the trimethoprim-induced secondary metabolome of Burkholderia thailandensis. ACS Chem Biol 11, 2124-2130.
DOI URL |
| [38] |
Osbourn A (2010). Secondary metabolic gene clusters: evolutionary toolkits for chemical innovation. Trends Genet 26, 449-457.
DOI PMID |
| [39] |
Park HL, Kim TL, Bhoo SH, Lee TH, Lee SW, Cho MH (2018). Biochemical characterization of the rice cinnamyl alcohol dehydrogenase gene family. Molecules 23, 2659.
DOI URL |
| [40] |
Qi X, Bakht S, Qin B, Leggett M, Hemmings A, Mellon F, Eagles J, Werck-Reichhart D, Schaller H, Lesot A, Melton R, Osbourn A (2006). A different function for a member of an ancient and highly conserved cytochrome P450 family: from essential sterols to plant defense. Proc Natl Acad Sci USA 103, 18848-18853.
DOI PMID |
| [41] |
Qi XQ, Bakht S, Leggett M, Maxwell C, Melton R, Osbourn A (2004). A gene cluster for secondary metabolism in oat: implications for the evolution of metabolic diversity in plants. Proc Natl Acad Sci USA 101, 8233-8238.
PMID |
| [42] |
Roddick JG, Weissenberg M, Leonard AL (2001). Membrane disruption and enzyme inhibition by naturally-occurring and modified chacotriose-containing Solanum steroidal glycoalkaloids. Phytochemistry 56, 603-610.
PMID |
| [43] |
Rokas A, Mead ME, Steenwyk JL, Raja HA, Oberlies NH (2020). Biosynthetic gene clusters and the evolution of fungal chemodiversity. Nat Prod Rep 37, 868-878.
DOI PMID |
| [44] |
Rokas A, Wisecaver JH, Lind AL (2018). The birth, evolution and death of metabolic gene clusters in fungi. Nat Rev Microbiol 16, 731-744.
DOI PMID |
| [45] | Schneider LM, Adamski NM, Christensen CE, Stuart DB, Vautrin S, Hansson M, Uauy C, von Wettstein-Knowles P (2016). The Cer-cqu gene cluster determines three key players in a β-diketone synthase polyketide pathway synthesizing aliphatics in epicuticular waxes. J Exp Bot 67, 2715-2730. |
| [46] |
Shang Y, Ma YS, Zhou Y, Zhang HM, Duan LX, Chen HM, Zeng JG, Zhou Q, Wang SH, Gu WJ, Liu M, Ren JW, Gu XF, Zhang SP, Wang Y, Yasukawa K, Bouwmeester HJ, Qi XQ, Zhang ZH, Lucas WJ, Huang SW (2014). Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 346, 1084-1088.
DOI PMID |
| [47] |
Shen SQ, Peng M, Fang H, Wang ZX, Zhou S, Jing XY, Zhang M, Yang CK, Guo H, Li YF, Lei L, Shi YH, Sun YY, Liu XQ, Xu CP, Tohge T, Yuan M, Fernie AR, Ning YS, Wang GL, Luo J (2021). An Oryza-specific hydroxycinnamoyl tyramine gene cluster contributes to enhanced disease resistance. Sci Bull 66, 2369-2380.
DOI URL |
| [48] | Smith DJ, Burnham MKR, Edwards J, Earl AJ, Turner G (1990). Cloning and heterologous expression of the penicillin biosynthetic gene cluster from Penicillium chrysogenum. Biotechnology 8, 39-41. |
| [49] |
Takos AM, Knudsen C, Lai D, Kannangara R, Mikkelsen L, Motawia MS, Olsen CE, Sato S, Tabata S, Jørgensen K, Møller BL, Rook F (2011). Genomic clustering of cyanogenic glucoside biosynthetic genes aids their identification in Lotus japonicus and suggests the repeated evolution of this chemical defence pathway. Plant J 68, 273-286.
DOI URL |
| [50] |
Tao HY, Zuo L, Xu HL, Li C, Qiao G, Guo MY, Lin XK (2020). Alkaloids as anticancer agents: a review of Chinese patents in recent 5 years. Recent Pat Anticancer Drug Discov 15, 2-13.
DOI PMID |
| [51] | Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Høj PB, Møller BL (2001). Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293, 1826-1828. |
| [52] |
Tunyasuvunakool K, Adler J, Wu Z, Green T, Zielinski M, Žídek A, Bridgland A, Cowie A, Meyer C, Laydon A, Velankar S, Kleywegt GJ, Bateman A, Evans R, Pritzel A, Figurnov M, Ronneberger O, Bates R, Kohl SAA, Potapenko A, Ballard AJ, Romera-Paredes B, Nikolov S, Jain R, Clancy E, Reiman D, Petersen S, Senior AW, Kavukcuoglu K, Birney E, Kohli P, Jumper J, Hassabis D (2021). Highly accurate protein structure prediction for the human proteome. Nature 596, 590-596.
DOI |
| [53] |
Varshney RK, Bohra A, Yu JM, Graner A, Zhang QF, Sorrells ME (2021). Designing future crops: genomics- assisted breeding comes of age. Trends Plant Sci 26, 631-649.
DOI PMID |
| [54] |
Verpoorte R, Memelink J (2002). Engineering secondary metabolite production in plants. Curr Opin Biotechnol 13, 181-187.
DOI URL |
| [55] |
von Rad U, Hüttl R, Lottspeich F, Gierl A, Frey M (2001). Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize. Plant J 28, 633-642.
PMID |
| [56] |
Vranová E, Coman D, Gruissem W (2013). Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol 64, 665-700.
DOI PMID |
| [57] |
Weber T, Kim HU (2016). The secondary metabolite bioinformatics portal: computational tools to facilitate synthetic biology of secondary metabolite production. Synth Syst Biotechnol 1, 69-79.
DOI PMID |
| [58] |
Winzer T, Gazda V, He ZS, Kaminski F, Kern M, Larson TR, Li Y, Meade F, Teodor R, Vaistij FE, Walker C, Bowser TA, Graham IA (2012). A Papaver somniferum 10-gene cluster for synthesis of the anticancer alkaloid noscapine. Science 336, 1704-1708.
DOI PMID |
| [59] |
Wu X, Feng H, Wu D, Yan SJ, Zhang P, Wang WB, Zhang J, Ye JL, Dai GX, Fan Y, Li WK, Song BX, Geng ZD, Yang WL, Chen GX, Qin F, Terzaghi W, Stitzer M, Li L, Xiong LZ, Yan JB, Buckler E, Yang WN, Dai MQ (2021). Using high-throughput multiple optical phenotyping to decipher the genetic architecture of maize drought tolerance. Genome Biol 22, 185.
DOI PMID |
| [60] |
Wu S, Morotti AL, Wang SS, Wang Y, Xu XY, Chen JH, Wang GD, Tatsis EC (2022). Convergent gene clusters underpin hyperforin biosynthesis in St John’s wort. New Phytol 235, 646-661.
DOI URL |
| [61] |
Xu MM, Galhano R, Wiemann P, Bueno E, Tiernan M, Wu W, Chung IM, Gershenzon J, Tudzynski B, Sesma A, Peters RJ (2012). Genetic evidence for natural product-mediated plant-plant allelopathy in rice (Oryza sativa). New Phytol 193, 570-575.
DOI PMID |
| [62] |
Yamamuro C, Zhu JK, Yang ZB (2016). Epigenetic modifications and plant hormone action. Mol Plant 9, 57-70.
DOI PMID |
| [63] |
Yang WN, Feng H, Zhang XH, Zhang J, Doonan JH, Batchelor WD, Xiong LZ, Yan JB (2020). Crop phenomics and high-throughput phenotyping: past decades, current challenges, and future perspectives. Mol Plant 13, 187-214.
DOI PMID |
| [64] |
Yang XF, Gao SH, Guo L, Wang B, Jia YY, Zhou J, Che YZ, Jia P, Lin JD, Xu T, Sun JY, Ye K (2021). Three chromosome-scale Papaver genomes reveal punctuated patchwork evolution of the morphinan and noscapine biosynthesis pathway. Nat Commun 12, 6030.
DOI |
| [65] |
Yu N, Nützmann HW, MacDonald JT, Moore B, Field B, Berriri S, Trick M, Rosser SJ, Kumar SV, Freemont PS, Osbourn A (2016). Delineation of metabolic gene clusters in plant genomes by chromatin signatures. Nucleic Acids Res 44, 2255-2265.
DOI PMID |
| [66] |
Yue JP, Hu XY, Huang JL (2013). Horizontal gene transfer in the innovation and adaptation of land plants. Plant Signal Behav 8, e24130.
DOI URL |
| [67] |
Zhan CS, Lei L, Liu ZX, Zhou S, Yang CK, Zhu XT, Guo H, Zhang F, Peng M, Zhang M, Li YF, Yang ZX, Sun YY, Shi YH, Li K, Liu L, Shen SQ, Wang XY, Shao JW, Jing XY, Wang ZX, Li Y, Czechowski T, Hasegawa M, Graham I, Tohge T, Qu LH, Liu XQ, Fernie AR, Chen LL, Yuan M, Luo J (2020). Selection of a subspecies-specific diterpene gene cluster implicated in rice disease resistance. Nat Plants 6, 1447-1454.
DOI PMID |
| [68] |
Zhan CS, Shen SQ, Yang CK, Liu ZH, Fernie AR, Graham IA, Luo J (2022). Plant metabolic gene clusters in the multiomics era. Trends Plant Sci 27, 981-1001.
DOI URL |
| [69] |
Zhu GT, Wang SC, Huang ZJ, Zhang SB, Liao QG, Zhang CZ, Lin T, Qin M, Peng M, Yang CK, Cao X, Han X, Wang XX, van der Knaap E, Zhang ZH, Cui X, Klee H, Fernie AR, Luo J, Huang SW (2018). Rewiring of the fruit metabolome in tomato breeding. Cell 172, 249-261.
DOI PMID |
| [1] | 平晓燕, 杜毅倩, 赖仕蓉, 孔梦桥, 余国杰. 植物应对食草动物采食的化学防御策略研究进展[J]. 植物生态学报, 2025, 49(5): 667-680. |
| [2] | 赵常提, 夏青霖, 田地, 陈冰瑞, 朱瑞德, 刘宵含, 俞果, 吉成均. 长期氮添加对温带落叶阔叶林优势植物叶片次生代谢产物的影响[J]. 植物生态学报, 2024, 48(12): 1576-1588. |
| [3] | 张照宇, 王清芸, 石雷, 余文刚, 张永清, 崔洪霞. 丁香属次生代谢产物及其与系统演化和地理环境的关联[J]. 植物学报, 2021, 56(4): 470-479. |
| [4] | 方荣俊, 赵华, 廖永辉, 汤程贻, 吴凤瑶, 朱煜, 庞延军, 陆桂华, 王小明, 杨荣武, 戚金亮, 杨永华. 乙烯对植物次生代谢产物合成的双重调控效应[J]. 植物学报, 2014, 49(5): 626-639. |
| [5] | 苏文华, 张光飞, 周鸿, 张亚妮. 短葶飞蓬黄酮及咖啡酸酯的含量与土壤氮供应量的关系[J]. 植物生态学报, 2009, 33(5): 885-892. |
| [6] | 梁宗琦. 真菌次生代谢产物多样性及其潜在应用价值[J]. 生物多样性, 1999, 07(2): 145-150. |
| [7] | 梁宗琦. 虫生真菌的生物多样性[J]. 生物多样性, 1996, 04(4): 235-241. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||