

植物学报 ›› 2025, Vol. 60 ›› Issue (1): 81-89.DOI: 10.11983/CBB24022 cstr: 32102.14.CBB24022
收稿日期:2024-02-18
接受日期:2024-03-30
出版日期:2025-01-10
发布日期:2024-04-02
通讯作者:
* E-mail: fengmin@ibcas.ac.cn作者简介:徐秀苹, 中国科学院植物研究所正高级工程师, 植物形态与结构平台主管, 负责显微CT扫描系统和冷冻扫描电镜等大型仪器的运行、管理及功能开发。第一完成人授权发明专利3项、实用新型专利1项。以第一作者发表技术方法类文章4篇, 出版仪器图书1册。完成2项中国科学院仪器设备功能开发项目。入选2022年度中国科学院技术支撑人才项目
基金资助:
Xiuping Xu1,2, Xiaoyu Yang3,4, Min Feng1,2,*( )
)
Received:2024-02-18
Accepted:2024-03-30
Online:2025-01-10
Published:2024-04-02
Contact:
* E-mail: fengmin@ibcas.ac.cn摘要: 禾谷类种子淀粉胚乳是人类主食的重要来源, 但观察其成熟细胞形态的方法非常有限。显微CT是一种非破坏性的三维成像技术, 是研究植物形态的有力工具。由于成熟禾谷类种子淀粉胚乳密度均匀, 常规显微CT技术无法应用。在前期研究的基础上, 用磷钨酸处理10种不同禾谷类作物种子, 经CO2临界点干燥后, 再用显微CT扫描, 可以清晰地分辨出禾谷类作物淀粉胚乳的细胞结构, 为研究此类细胞结构提供了一种新方法。
徐秀苹, 杨小雨, 冯旻. 成熟禾谷类作物种子胚乳细胞的显微CT扫描方法. 植物学报, 2025, 60(1): 81-89.
Xiuping Xu, Xiaoyu Yang, Min Feng. A New Cereal Seed Treatment Method for Displaying Endosperm Cell Structures Under Micro CT Scanning. Chinese Bulletin of Botany, 2025, 60(1): 81-89.
| Seed | Voltage (kV) | Resolution (µm) |
|---|---|---|
| Rice | 100 | 0.88 |
| Rice (partially enlarged) | 50 | 0.56 |
| Purple rice | 100 | 0.95 |
| Red rice | 100 | 0.74 |
| Glutinous rice | 100 | 0.88 |
| Millet | 41 | 0.68 |
| Oats | 100 | 0.88 |
| Sorghum | 100 | 1.15 |
| Job’s tears | 100 | 2.60 |
| Corn | 100 | 1.48 |
| Corn (partially enlarged) | 49 | 0.56 |
| Wheat | 100 | 1.15 |
表1 成熟种子扫描电压和分辨率
Table 1 Scanning voltage and resolution of ripe seeds
| Seed | Voltage (kV) | Resolution (µm) |
|---|---|---|
| Rice | 100 | 0.88 |
| Rice (partially enlarged) | 50 | 0.56 |
| Purple rice | 100 | 0.95 |
| Red rice | 100 | 0.74 |
| Glutinous rice | 100 | 0.88 |
| Millet | 41 | 0.68 |
| Oats | 100 | 0.88 |
| Sorghum | 100 | 1.15 |
| Job’s tears | 100 | 2.60 |
| Corn | 100 | 1.48 |
| Corn (partially enlarged) | 49 | 0.56 |
| Wheat | 100 | 1.15 |
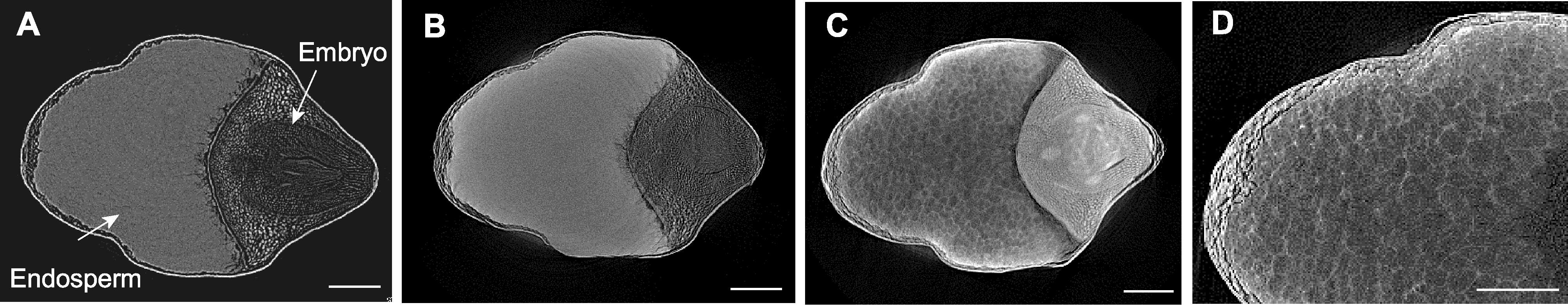
图1 不同处理下大米显微CT扫描 (A) FAA处理后的大米; (B) 10%碘化铯处理后的大米; (C) 10%磷钨酸处理后的大米; (D) 10%磷钨酸处理后的大米局部放大。Bars=500 µm
Figure 1 Micro CT scanning of rice seeds by different treatments (A) Rice seed treated by FAA; (B) Rice seed treated by 10% cesium iodide; (C) Rice seed treated by 10% phosphotungstic acid; (D) Enlarged view of rice seed treated by 10% phosphotungstic acid. Bars=500 µm
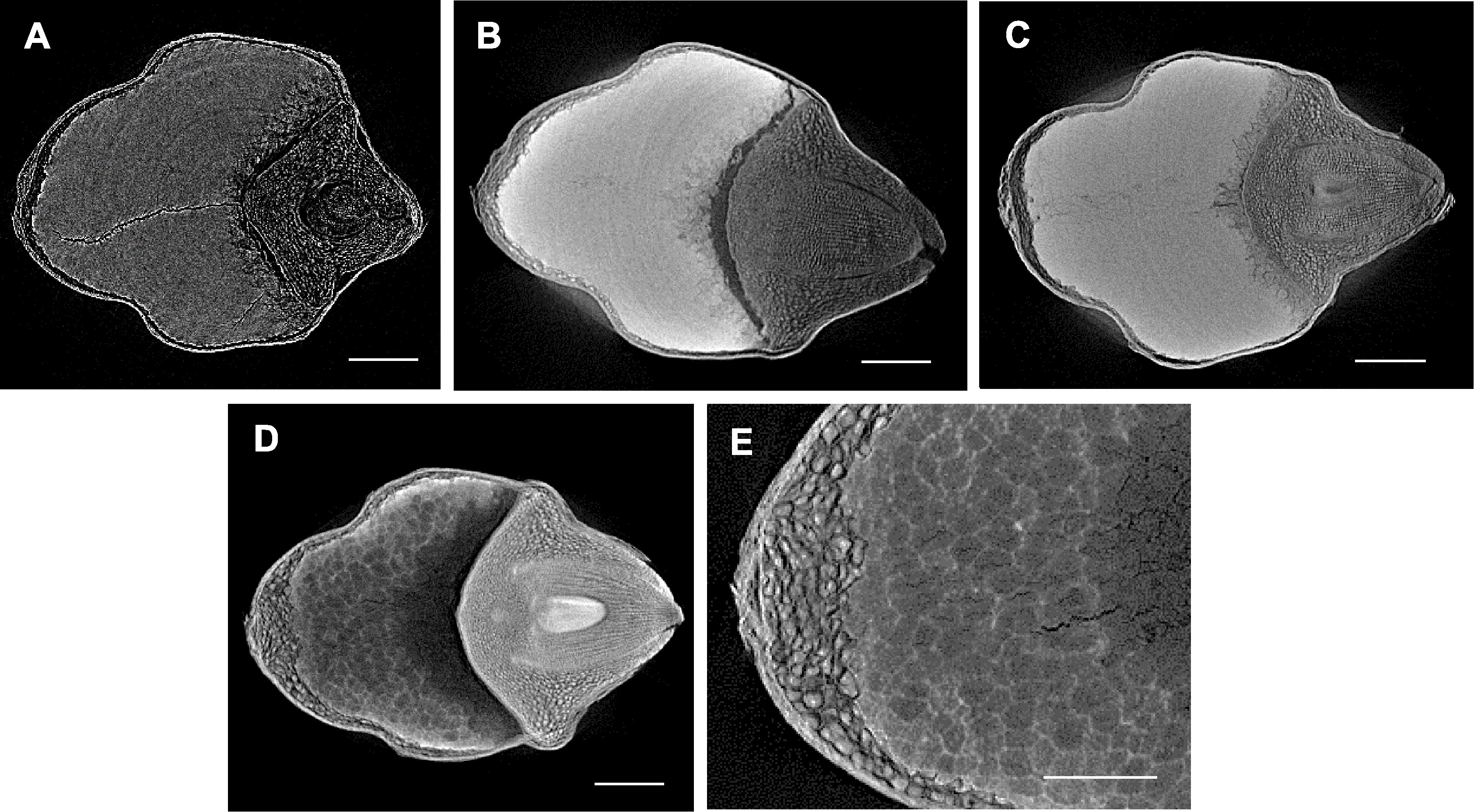
图2 不同处理下紫米显微CT扫描 (A) FAA处理后的紫米; (B) 10%碘化铯处理后的紫米; (C) Lugols溶液处理后的紫米; (D) 10%磷钨酸处理后的紫米; (E) 10%磷钨酸处理后的紫米局部放大。Bars=400 µm
Figure 2 Micro CT scanning of purple rice seeds by different treatments (A) Purple rice seed treated by FAA; (B) Purple rice seed treated by 10% cesium iodide; (C) Purple rice seed treated by Lugols solution; (D) Purple rice seed treated by 10% phosphotungstic acid; (E) Enlarged view of purple rice seed treated by 10% phosphotungstic acid. Bars=400 µm
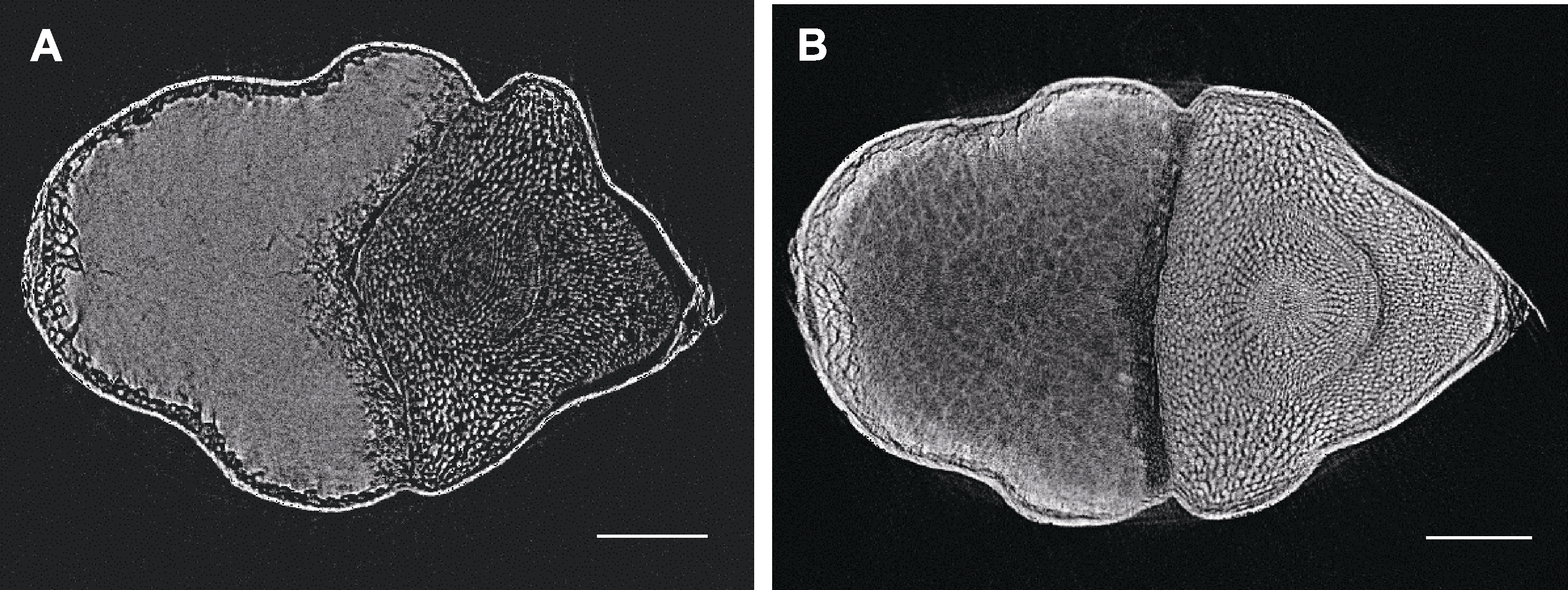
图3 不同处理下红米显微CT扫描 (A) FAA处理后的红米; (B) 10%磷钨酸处理后的红米。Bars=400 µm
Figure 3 Micro CT scanning of red rice seeds by different treatments (A) Red rice seed treated by FAA; (B) Red rice seed treated by 10% phosphotungstic acid. Bars=400 µm
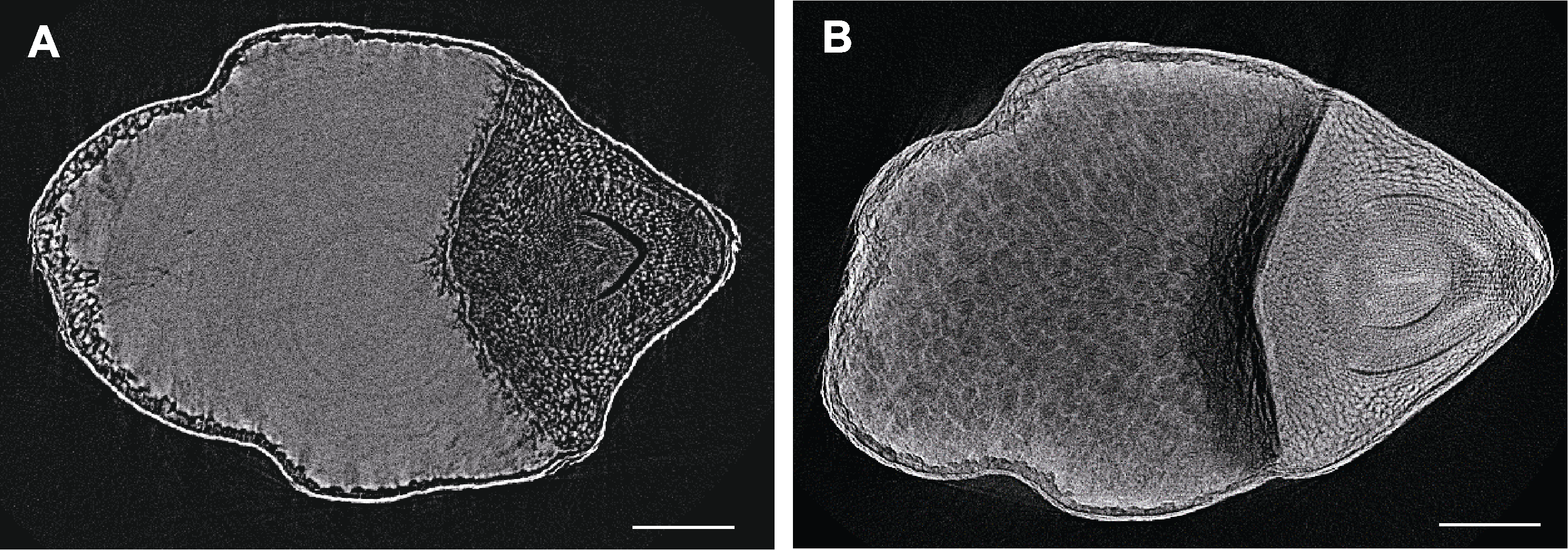
图4 不同处理下糯米显微CT扫描 (A) FAA处理后的糯米; (B) 10%磷钨酸处理后的糯米。Bars=400 µm
Figure 4 Micro CT scanning of polished glutinous rice seeds by different treatments (A) Polished glutinous rice seed treated by FAA; (B) Polished glutinous rice seed treated by 10% phosphotungstic acid. Bars=400 µm
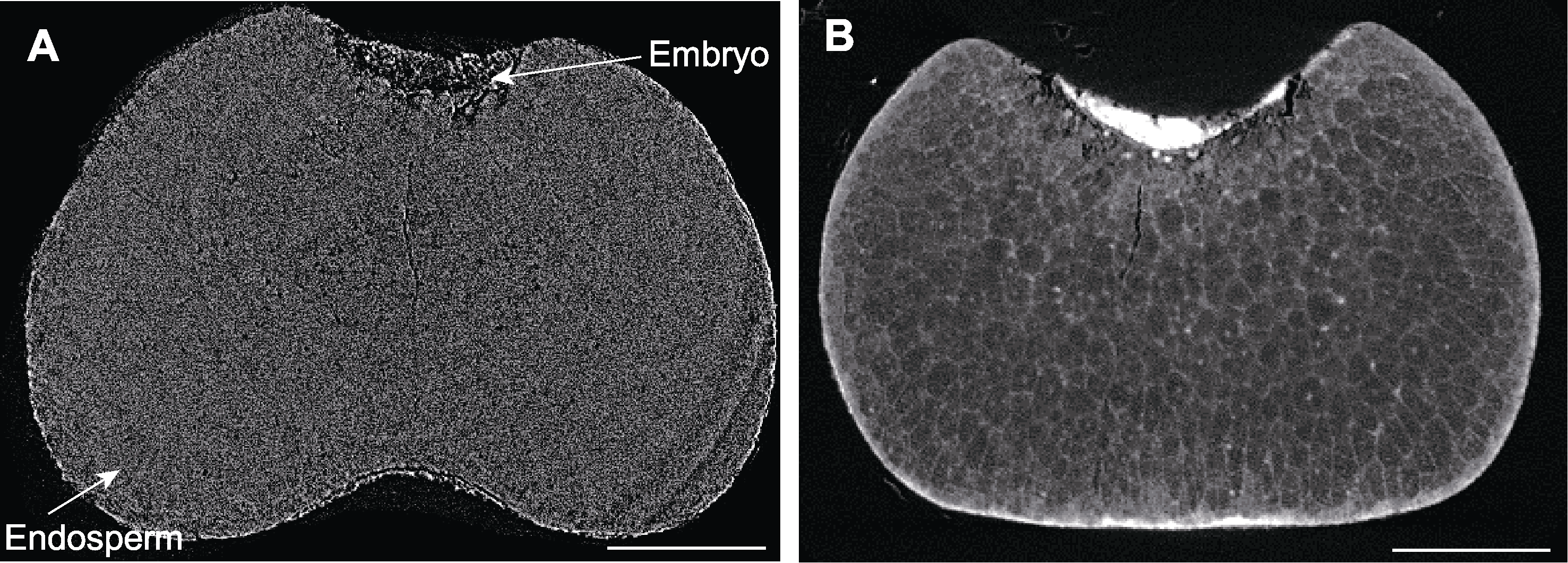
图5 不同处理下谷子种子显微CT扫描 (A) FAA处理后的谷子种子; (B) 10%磷钨酸处理后的谷子种子。Bars=300 µm
Figure 5 Micro CT scanning of millet seeds by different treatments (A) Millet seed treated by FAA; (B) Millet seed treated by 10% phosphotungstic acid. Bars=300 µm
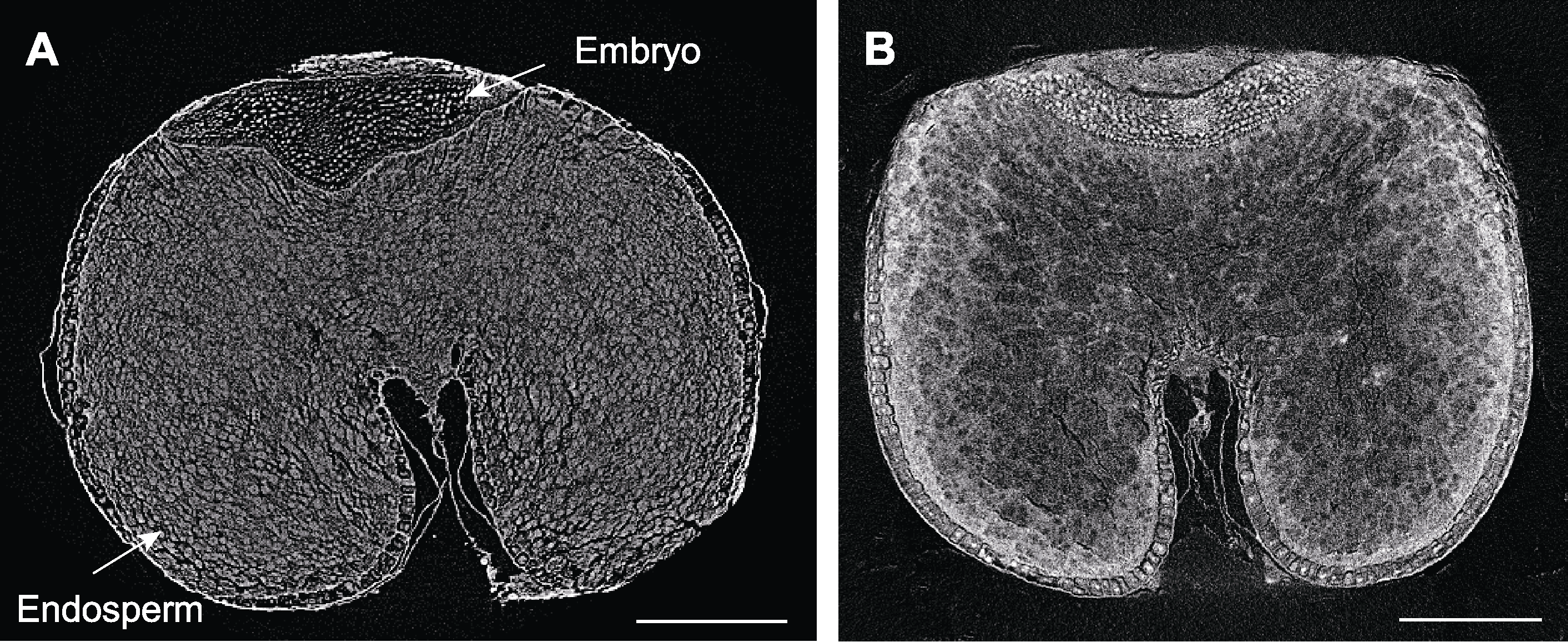
图6 不同处理下燕麦种子显微CT扫描 (A) FAA处理后的燕麦种子; (B) 10%磷钨酸处理后的燕麦种子。Bars=500 µm
Figure 6 Micro CT scanning of oat seeds by different treatments (A) Oat seed treated by FAA; (B) Oat seed treated by 10% phosphotungstic acid. Bars=500 µm
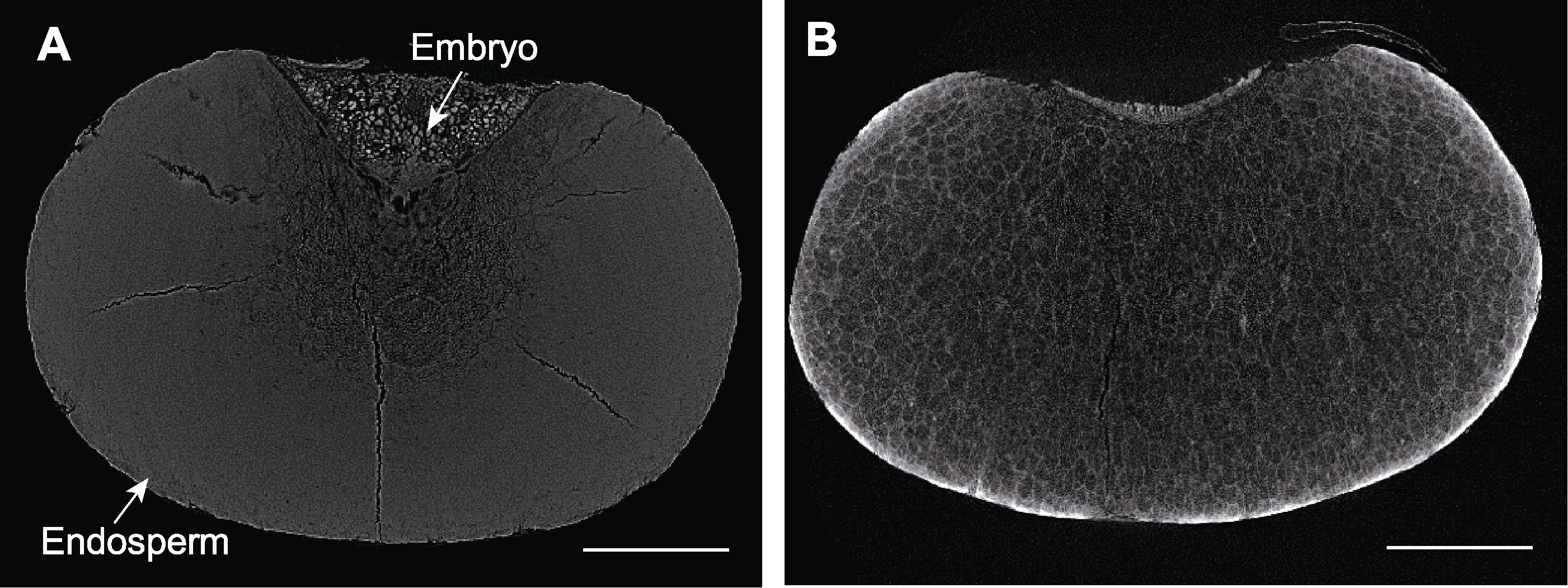
图7 不同处理下高粱种子显微CT扫描 (A) FAA处理后的高粱种子; (B) 10%磷钨酸处理后的高粱种子。Bars=500 µm
Figure 7 Micro CT scanning of sorghum seeds by different treatments (A) Sorghum seed treated by FAA; (B) Sorghum seed treated by 10% phosphotungstic acid. Bars=500 µm
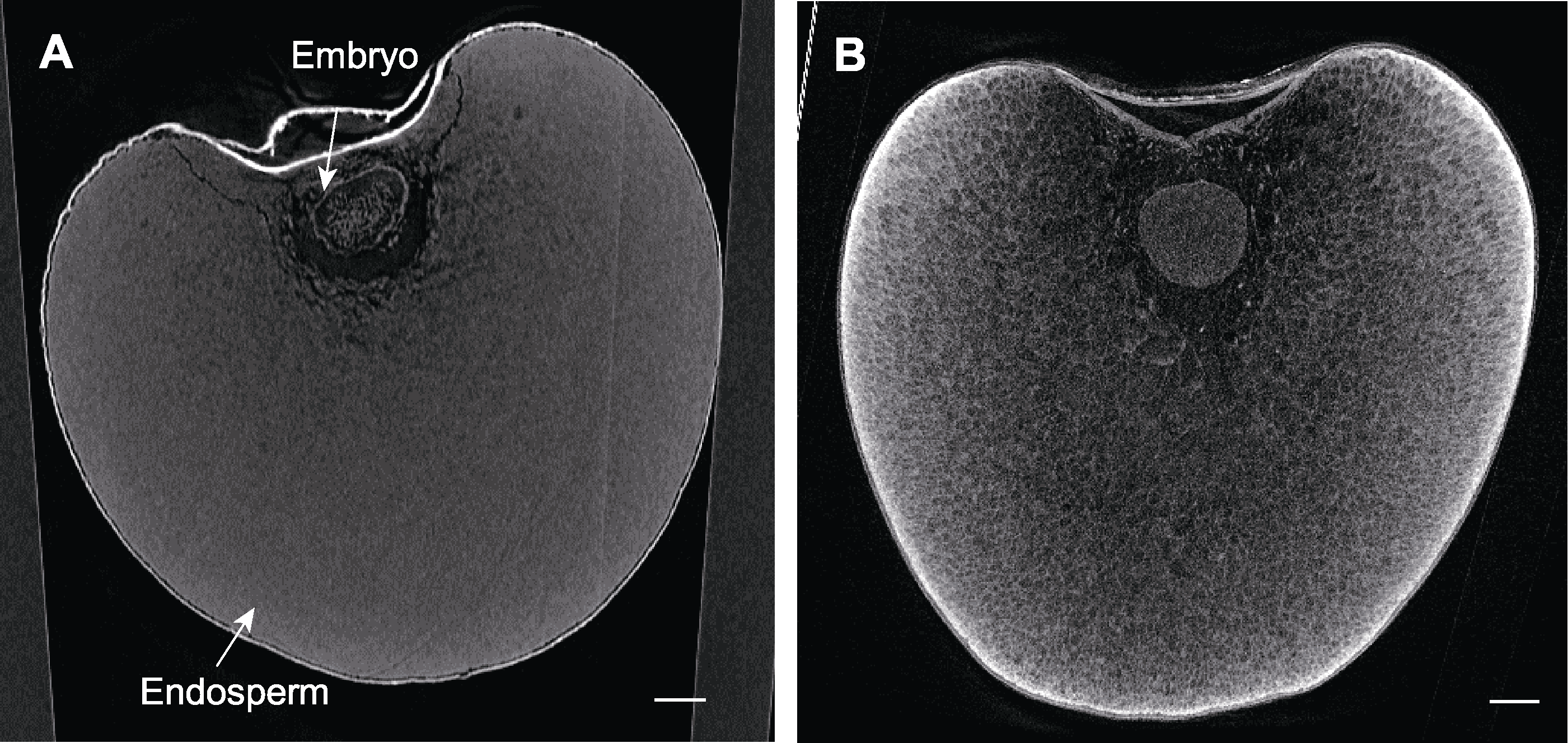
图8 不同处理下薏米种子显微CT扫描 (A) FAA处理后的薏米种子; (B) 10%磷钨酸处理后的薏米种子。Bars=500 µm
Figure 8 Micro CT scanning of the seeds of Job’s tears by different treatments (A) The seed of Job’s tears treated by FAA; (B) The seed of Job’s tears treated by 10% phosphotungstic acid. Bars=500 µm
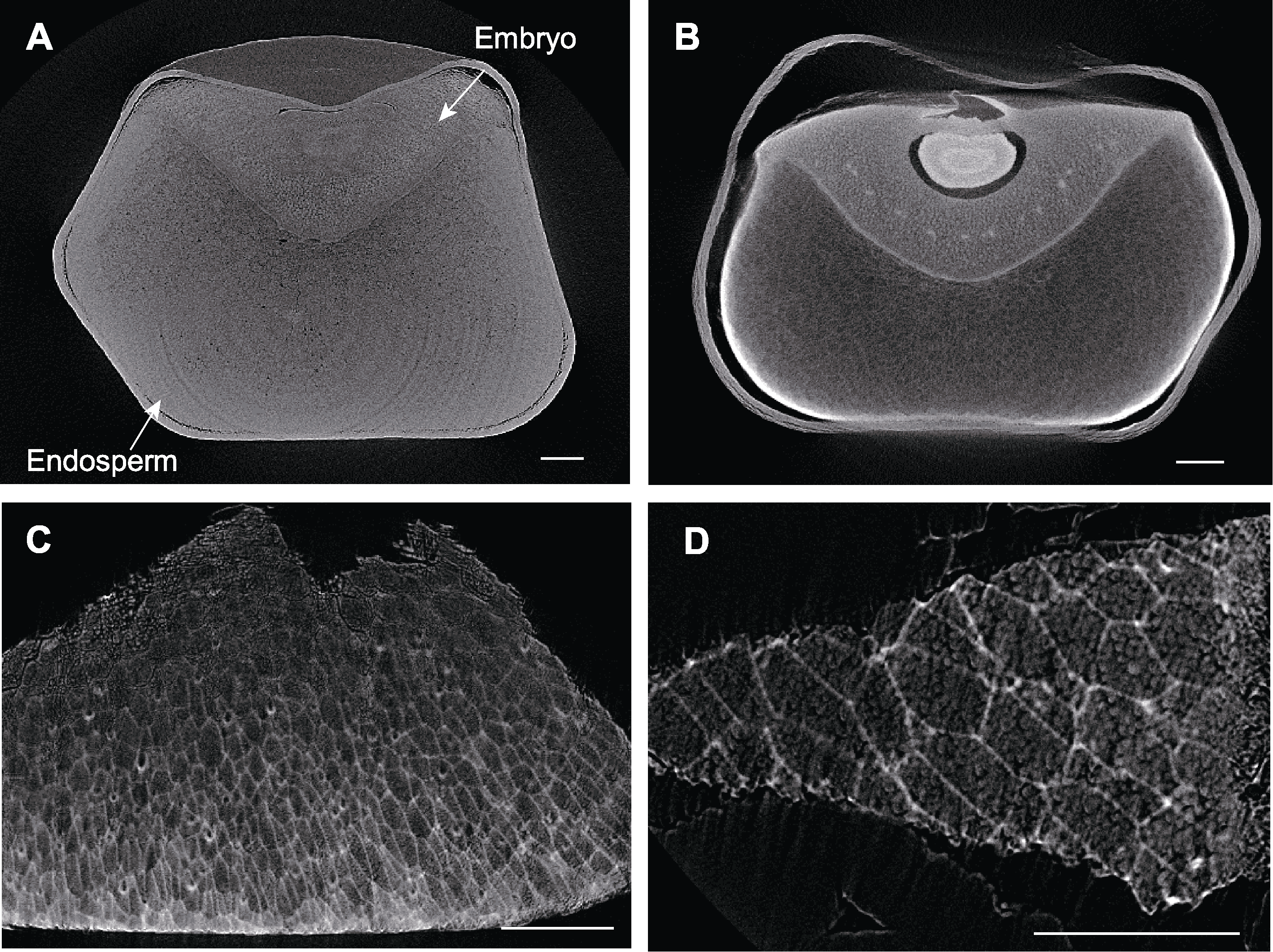
图9 不同处理下玉米种子显微CT扫描 (A) FAA处理后的玉米种子; (B) 10%磷钨酸处理后的玉米种子; (C) 10%磷钨酸处理后的玉米种子小块; (D) 10%磷钨酸处理后的玉米种子小块局部放大。Bars=400 µm
Figure 9 Micro CT scanning of maize seeds by different treatments (A) Maize seed treated by FAA; (B) Maize seed treated by 10% phosphotungstic acid; (C) Part of maize seed treated by 10% phosphotungstic acid; (D) Enlarged view of part of maize seed treated by 10% phosphotungstic acid. Bars=400 µm
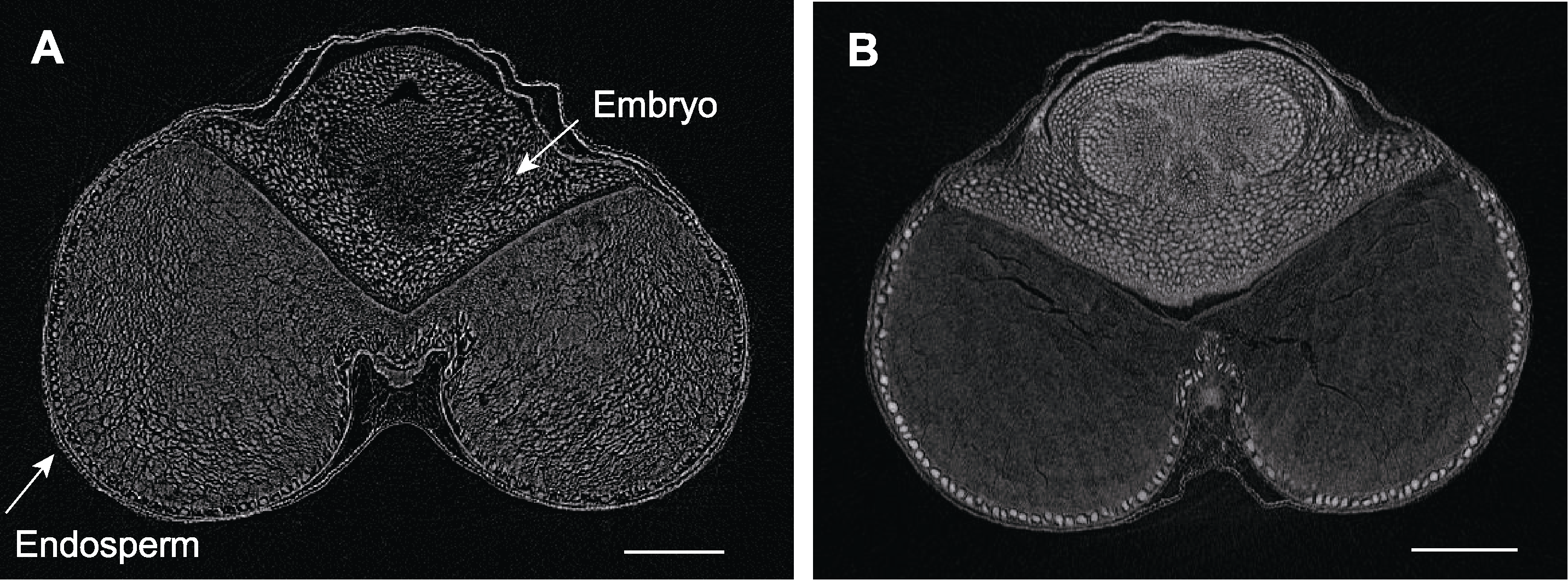
图10 不同处理下小麦种子显微CT扫描 (A) FAA处理后的小麦种子; (B) 10%磷钨酸处理后的小麦种子。Bars=500 µm
Figure 10 Micro CT scanning of wheat seeds by different treatments (A) Wheat seed treated by FAA; (B) Wheat seed treated by 10% phosphotungstic acid. Bars=500 µm
| Kind of seeds | Rice | Purple rice | Red rice | Glutinous rice | Millet (remove seed coat) | Oats | Sorghum (remove seed coat) | Job’s tears | Corn (remove seed coat) | Wheat |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment time (day) | 40 | 40 | 40 | 40 | 30 | 60 | 70 | 90 | 90 | 180 |
| Valid or not | Valid | Valid | Valid | Valid | Valid | Valid | Valid | Valid | Valid | Invalid |
表2 磷钨酸处理禾谷类种子胚乳效果
Table 2 Effect of cereal seed endosperm by phosphotungstic acid treatment
| Kind of seeds | Rice | Purple rice | Red rice | Glutinous rice | Millet (remove seed coat) | Oats | Sorghum (remove seed coat) | Job’s tears | Corn (remove seed coat) | Wheat |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment time (day) | 40 | 40 | 40 | 40 | 30 | 60 | 70 | 90 | 90 | 180 |
| Valid or not | Valid | Valid | Valid | Valid | Valid | Valid | Valid | Valid | Valid | Invalid |
| [1] |
Becraft PW, Gutierrez-Marcos J (2012). Endosperm development: dynamic processes and cellular innovations underlying sibling altruism. WIREs Dev Biol 1, 579-593.
DOI PMID |
| [2] | Hu J, Guan YJ (2022). Seed Biology, 2nd edn. Beijing: Higher Education Press. pp. 62-63. (in Chinese) |
| 胡晋, 关亚静 (2022). 种子生物学(第2版). 北京: 高等教育出版社. pp. 62-63. | |
| [3] | Huang LJ, Fu WL (2021). A water drop-shaped slingshot in plants: geometry and mechanics in the explosive seed dispersal of Orixa japonica (Rutaceae). Ann Bot 127, 765-774. |
| [4] | Kaestner A, Schneebeli M, Graf F (2006). Visualizing three-dimensional root networks using computed tomography. Geoderma 136, 459-469. |
| [5] |
Knipfer T, Reyes C, Earles JM, Berry ZC, Johnson DM, Brodersen CR, McElrone AJ (2019). Spatiotemporal coupling of vessel cavitation and discharge of stored xylem water in a tree sapling. Plant Physiol 179, 1658-1668.
DOI PMID |
| [6] | Korte N, Porembski S (2012). A morpho-anatomical characterisation of Myrothamnus moschatus (Myrothamnaceae) under the aspect of desiccation tolerance. Plant Biol 14, 537-541. |
| [7] | Liao H, Fu XH, Zhao HQ, Cheng J, Zhang R, Yao X, Duan XS, Shan HY, Kong HZ (2020). The morphology, molecular development and ecological function of pseudonectaries on Nigella damascena (Ranunculaceae) petals. Nat Commun 11, 1777. |
| [8] | Lin JX, Hu ZJ, Zhang X, Yang SY, Shan XY (2019). Method for Improving Imaging Quality of Arabidopsis thaliana Seeds. Chinese Patent, CN109142398A. 2019-01-04. (in Chinese) |
| 林金星, 胡子建, 张曦, 杨舜垚, 单晓昳 (2019). 一种提高拟南芥种子成像质量的方法. 中国专利, CN109142398A. 2019-01-04. | |
| [9] | Losso A, Bär A, Dämon B, Dullin C, Ganthaler A, Petruzzellis F, Savi T, Tromba G, Nardini A, Mayr S, Beikircher B (2019). Insights from in vivo micro-CT analysis: testing the hydraulic vulnerability segmentation in Acer pseudoplatanus and Fagus sylvatica seedlings. New Phytol 221, 1831-1842. |
| [10] | Perret JS, Al-Belushi ME, Deadman M (2007). Non-destructive visualization and quantification of roots using computed tomography. Soil Biol Biochem 39, 391-399. |
| [11] | Snell P, Wilkinson M, Taylor GJ, Hall S, Sharma S, Sirijovski N, Hansson M, Shewry PR, Hofvander P, Grimberg Å (2022). Characterisation of grains and flour fractions from field grown transgenic oil-accumulating wheat expressing oat WRI1. Plants 11, 889. |
| [12] | Staedler YM, Masson D, Schönenberger J (2013). Plant tissues in 3D via X-ray tomography: simple contrasting methods allow high resolution imaging. PLoS One 8, e75295. |
| [13] | Xu XP, Meng SC, Liang RH, Jin WQ, Feng M (2021). New method of improving micro-CT images contrasts on plant samples. J Chin Electron Microsc Soc 40, 460-466. (in Chinese) |
| 徐秀苹, 孟淑春, 梁荣花, 靳婉青, 冯旻 (2021). 磷钨酸和干燥处理提高植物样品显微CT成像对比度的方法. 电子显微学报 40, 460-466. | |
| [14] | Zhang CQ, Li Y (2019). Crop Seed Science, 2nd edn. Beijing: China Agriculture Press. pp. 16-18. (in Chinese) |
| 张春庆, 李岩 (2019). 作物种子学(第2版). 北京: 中国农业出版社. pp. 16-18. | |
| [15] |
Zhang X, Hu ZJ, Guo YY, Shan XY, Li XJ, Lin JX (2020). High-efficiency procedure to characterize, segment, and quantify complex multicellularity in raw micrographs in plants. Plant Methods 16, 100.
DOI PMID |
| [1] | 刘慧强, 凯撒·苏来曼, 孙芸, 庞渊, 樊孝喜, 谢茹, 柳超, 段颖妮, 马燕. 新疆阿魏特征显微结构的三维原位无损研究[J]. 植物学报, 2018, 53(3): 364-371. |
| [2] | 沈漫. 两相法分离地被菊叶片质膜的研究[J]. 植物学报, 2004, 21(01): 66-73. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||