

植物学报 ›› 2017, Vol. 52 ›› Issue (5): 608-614.DOI: 10.11983/CBB16196 cstr: 32102.14.CBB16196
姚宏伟1, 刘洋2, 程宇来2, 于海洋3, 刘志亮3, 杨菊1,*( )
)
收稿日期:2016-10-10
接受日期:2017-03-07
出版日期:2017-09-01
发布日期:2017-07-10
通讯作者:
杨菊
基金资助:Hongwei Yao1, Yang Liu2, Yulai Cheng2, Haiyang Yu3, Zhiliang Liu3, Ju Yang1
Received:2016-10-10
Accepted:2017-03-07
Online:2017-09-01
Published:2017-07-10
Contact:
Ju Yang
摘要: 以拟南芥(Arabidopsis thaliana)和谷子(Setaria italic)为研究材料, 利用锌特异性探针HL1, 使用荧光分光光度仪、等温滴定热量测定仪(ITC200)和倒置荧光显微镜等仪器探究了该化学探针的特性以及植物细胞外游离Zn2+的分布。结果表明, 当HL1与不同元素溶液混合时, 只与Zn2+特异性结合, 在紫外光(UV)激发下, 发射出波长为500 nm的蓝色荧光; 生成物的平衡解离常数KD=7.02×10-4 mol·L-1, 具有很好的稳定性。拟南芥叶片中的Zn2+分布于细胞间隙及叶表皮毛的外周和表层, 且叶表皮毛的荧光强度具有明显的浓度依赖性; 谷子叶片中的Zn2+分布在细胞间隙以及维管组织。拟南芥根中的Zn2+分布于根的伸长区, 且荧光强度也明显地表现出与浓度相关。由此推断, 根伸长区与Zn2+运输有关, 叶的维管组织是植物细胞外运输Zn2+的主要途径, 细胞间隙和叶表皮毛是植物储存Zn2+的主要区域。HL1适用于检测细胞外Zn2+的分布。
姚宏伟, 刘洋, 程宇来, 于海洋, 刘志亮, 杨菊. 利用锌特异性探针HL1示踪植物细胞外Zn2+的分布. 植物学报, 2017, 52(5): 608-614.
Hongwei Yao, Yang Liu, Yulai Cheng, Haiyang Yu, Zhiliang Liu, Ju Yang. Fluorescence Imaging of the Extracellular Zinc Distribution in Plants by Using a Highly Specific Fluorescent Probe. Chinese Bulletin of Botany, 2017, 52(5): 608-614.
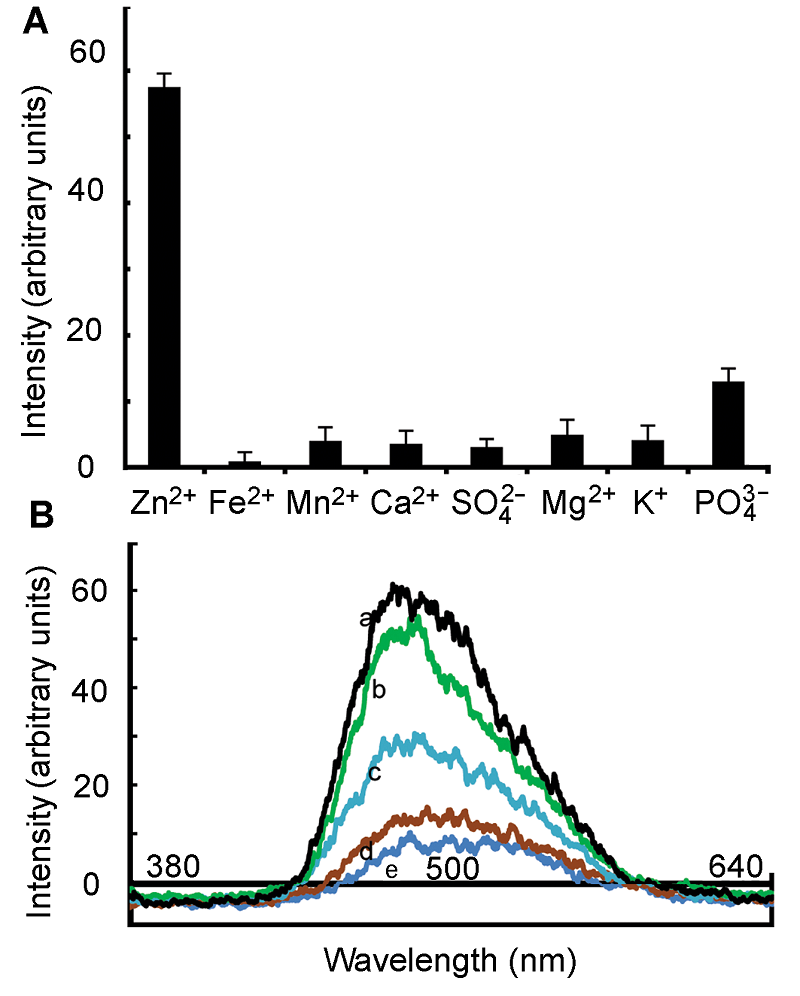
图2 HL1的特异性及对Zn2+结合的敏感性(A) 1 mmol·L-1不同元素与HL1探针结合后在500 nm处的荧光强度; (B) 不同Zn2+浓度下的荧光强度(a: 1 mmol∙L-1 Zn2+; b: 0.5 mmol∙L-1 Zn2+; c: 0.1 mmol∙L-1 Zn2+; d: 0.05 mmol∙L-1 Zn2+; e: 0.01 mmol∙L-1 Zn2+)
Figure 2 Specific and sensitivity of combination of HL1 and Zn2+(A) Fluorescence intensity of different element (1 mmol∙L-1) in presence of ligand HL1 (1 mmol∙L-1) at 500 nm; (B) Plot of fluorescence intensity against different concentration of Zn2+ at 500 nm (a: 1 mmol∙L-1 Zn2+; b: 0.5 mmol∙L-1 Zn2+; c: 0.1 mmol∙L-1 Zn2+; d: 0.05 mmol∙L-1 Zn2+; e: 0.01 mmol∙L-1 Zn2+)
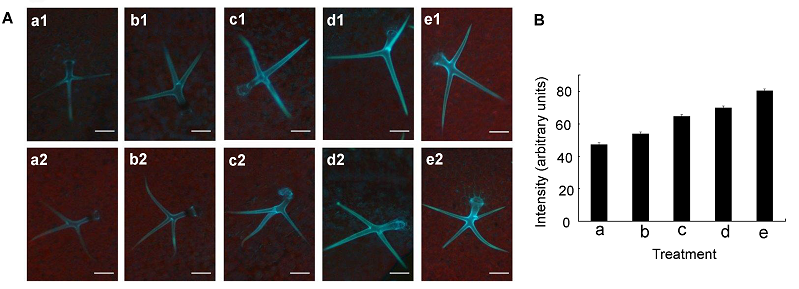
图4 拟南芥叶细胞外Zn2+分布荧光图像(A) 用不同浓度ZnSO4溶液及HL1溶液处理成熟的拟南芥叶片, a1和a2为对照, b1和b2处理ZnSO4溶液浓度为0.3 μmol∙L-1, c1和c2处理ZnSO4溶液浓度为3 μmol∙L-1, d1和d2处理ZnSO4溶液浓度为30 μmol∙L-1, e1和e2处理ZnSO4溶液浓度为300 μmol∙L-1; (B) 不同浓度ZnSO4溶液及HL1溶液处理下的拟南芥叶表皮毛的荧光强度, 其中a为对照, b、c、d和e分别为叶表皮毛在Zn2+浓度为0.3、3、30和300 μmol∙L-1时的荧光强度。蓝色荧光代表Zn2+, 红色荧光代表叶绿素。Bar=100 μm
Figure 4 Fluorescence imaging showing the distribution of extracellular Zn2+ in Arabidopsis leaves(A) Mature Arabidopsis leaves pre-treated with different concentrations of ZnSO4 and HL1 (1 mmol∙L-1) for 4 h, a1 and a2 for control, b1 and b2 for 0.3 μmol∙L-1 ZnSO4, c1 and c2 for 3 μmol∙L-1 ZnSO4, d1 and d2 for 30 μmol∙L-1 ZnSO4, e1 and e2 for 300 μmol∙L-1 ZnSO4; (B) The fluorescence intensity of trichome in Arabidopsis leaf with different concentration of ZnSO4 and HL1 (1 mmol∙L-1) for 4 h, a for control, b for 0.3 μmol∙L-1 ZnSO4, c for 3 μmol∙L-1 ZnSO4, d for 30 μmol∙L-1 ZnSO4, and e for 300 μmol∙L-1 ZnSO4. Blue fluorescence represents Zn2+ and red fluorescence for chlorophyll. Bar=100 μm

图5 植物细胞在1 mmol∙L-1 HL1下的荧光图像蓝色荧光代表Zn2+, 红色荧光代表叶绿素。Bar=50 μm
Figure 5 Fluorescence image of plant cell in presence of 1 mmol∙L-1 HL1Blue represents Zn2+ and red for chlorophyll. Bar=50 μm
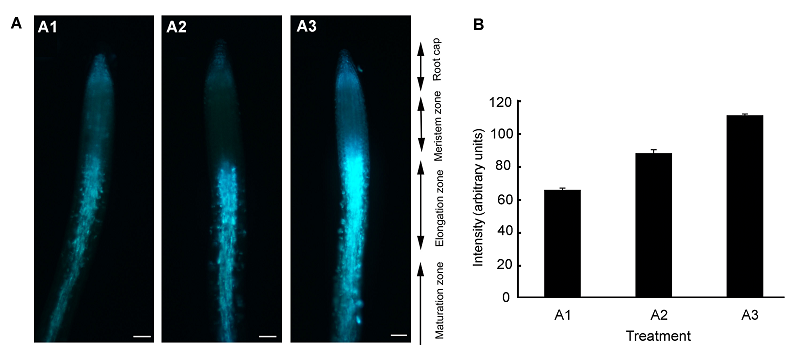
图6 拟南芥根细胞外Zn2+分布荧光图像(A) 分别用不同浓度的ZnSO4溶液与HL1溶液共处理2 cm左右的拟南芥根, 处理时间为4小时, 其中A1为对照, A2和A3中Zn2+浓度分别为30和300 μmol∙L-1 (Bar=100 μm); (B) A1、A2和A3分别对应处理液Zn2+浓度0、30和300 μmol∙L-1下的荧光强度。蓝色荧光代表Zn2+。
Figure 6 Fluorescence images showing the distribution of extracellular Zn2+ in Arabidopsis roots(A) Two-centimeter-long Arabidopsis root pre-treated with different concentrations of ZnSO4 and HL1 (1 mmol∙L-1) for 4 h, A1, A2, and A3 for control, 30 μmol∙L-1 ZnSO4 and 300 μmol∙L-1 ZnSO4, respectively (Bar=100 μm); (B) A1, A2, and A3 corresponding to the fluorescence intensity of Arabidopsis root treated with different concentrations of ZnSO4 for 0, 30, and 300 μmol∙L-1. Blue fluorescence represents Zn2+.
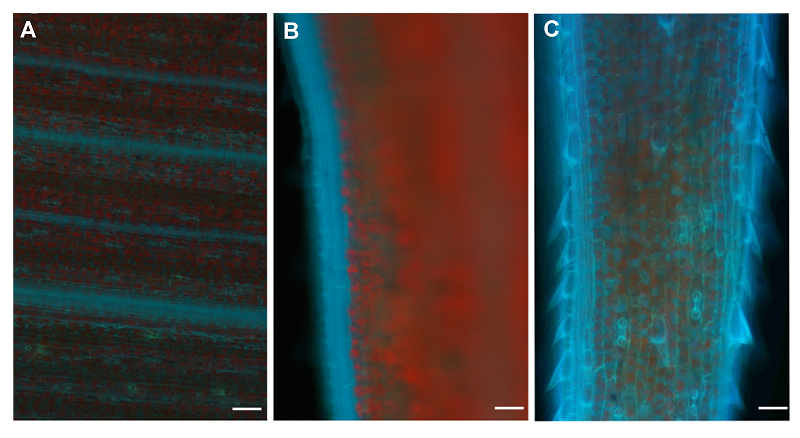
图7 谷子叶片细胞外Zn2+分布荧光图像(A) 叶中央; (B) 叶边缘; (C) 叶尖。蓝色荧光代表Zn2+, 红色荧光代表叶绿素。Bar=100 μm
Figure 7 Fluorescence images showing the distribution of extracellular Zn2+ in Setaria italic leaves(A) The center of leaf; (B) The margin of leaf; (C) The apex of leaf. Blue fluorescence represents Zn2+ and red fluorescence for chlorophyll. Bar=100 μm
| [1] | Bermejo C, Ewald JC, Lanquar V, Jones AM, Frommer WB (2011). In vivo biochemistry: quantifying ion and metabolite levels in individual cells orcultures of yeast.Biochem J 438, 1-10. |
| [2] | Cakmak I (2000). Tansley review No. 111: possible roles of zinc in protecting plant cells from damage by reactive oxygen species.New Phytol 146, 185-205. |
| [3] | Hacisalihoglu G, Hart JJ, Wang YH, Cakmak I, Kochian LV (2003). Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in whe- at.Plant Physiol 131, 595-602. |
| [4] | Hambidge KM, Miller LV, Westcott JE, Sheng XY, Krebs NF (2010). Zinc bioavailability and homeostasis.Am J Clin Nutr 91, 1478S-1483S. |
| [5] | Hantke K (2001). Bacterial zinc transporters and regulators. BioMetals 14, 239-249. |
| [6] | Hussain D, Haydon MJ, Wang YW, Wong E, Sherson SM, Young J, Camakaris J, Harper JF, Cobbett CS (2004). P-type ATPase heavy metal transporters with roles in es- sential zinc homeostasis in Arabidopsis.Plant Cell 16, 1327-1339. |
| [7] | Ji YF, Wang R, Ding S, Du CF, Liu ZL (2012). Synthesis, crystal structures and fluorescence studies of three new Zn(II) complexes with multidentate Schiff base ligands.In- organic Chem Commun 16, 47-50. |
| [8] | Kelleher SL, Lönnerdal B (2005). Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin.Cell Physiol 288, C1042-C1047. |
| [9] | Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P (2009). The analysis of Arabidopsis nicotiana- mine synthase mutants reveals functions for nicotiana- mine in seed iron loading and iron deficiency responses.Plant Physiol 150, 257-271. |
| [10] | Krämer U (2010). Metal hyperaccumulation in plants.Annu Rev Plant Biol 61, 517-534. |
| [11] | Lanquar V, Lelièvre F, Bolte S, Hamès C, Alcon C, Neumann D, Vansuyt G, Curie C, Schroder A, Krämer U, Barbier-Brygoo H, Thomine S (2005). Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron.EMBO J 24, 4041-4051. |
| [12] | Liuzzi JP, Cousins RJ (2004). Mammalian zinc transporters. Annu Rev Nutr 24, 151-172. |
| [13] | Maret W (2009). Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals.Biometals 22, 149-157. |
| [14] | Seigneurin-Berny D, Gravot A, Auroy P, Mazard C, Kraut A, Finazzi G, Grunwald D, Rappaport F, Vavasseur A, Joyard J, Richaud P, Rolland N (2006). HMA1, a new Cu-ATPase of the chloroplast envelope, is essential for growth under adverse light conditions.J Biol Chem 281, 2882-2892. |
| [15] | Sinclair SA, Krämer U (2012). The zinc homeostasis network of land plants.Biochim Biophys Acta 1823, 1553-1567. |
| [16] | Szymanski DB, Lloyd AM, Marks MD (2000). Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis.Trends Plant Sci 5, 214-219. |
| [17] | Zhao H, Eide D (1996). The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation.Proc Natl Acad Sci USA 93, 2454-2458. |
| [1] | 刘慧, 郭丹丽, 蔡大润, 黄先忠. 小拟南芥ApZFP基因异源超表达促进拟南芥开花并提高耐逆性[J]. 植物学报, 2016, 51(3): 296-305. |
| [2] | 苏晓娟, 樊保国, 袁丽钗, 崔秀娜, 卢善发. 实时荧光定量PCR分析中毛果杨内参基因的筛选和验证[J]. 植物学报, 2013, 48(5): 507-518. |
| [3] | 郭欣欣, 毛雪, 张敏. 锌对不同发育时期子代果蝇基因组DNA甲基化的影响[J]. 生物多样性, 2012, 20(6): 710-715. |
| [4] | 张现伟;杨莉;张涛;蒋开锋;王贵学;郑家奎;*;倪先林;田翠;曹应江. 水稻籽粒锌含量的QTL 定位[J]. 植物学报, 2009, 44(05): 594-600. |
| [5] | 王祥 李鹏 印莉萍. 酵母和植物的锌转运系统及其调控[J]. 植物学报, 2007, 24(06): 799-806. |
| [6] | 尹增芳 樊汝汶. 美洲黑杨次生维管组织发育的内在节律性与细胞内含物的动态变化[J]. 植物学报, 2006, 23(3): 262-268. |
| [7] | 聂湘平, 蓝崇钰, 束文圣, 张志权, 黄铭洪. 锌对大叶相思-根瘤菌共生固氮体系影响研究[J]. 植物生态学报, 2002, 26(3): 264-268. |
| [8] | 简曙光, 杨中艺. 茎瘤对长喙田菁在铅锌尾矿环境适应中的意义I. 茎瘤对长喙田菁生长发育的影响[J]. 植物生态学报, 2002, 26(1): 96-100. |
| [9] | 杨肖娥, 龙新宪, 倪吾钟, 倪士峰. 古老铅锌矿区生态型东南景天对锌耐性及超积累特性的研究[J]. 植物生态学报, 2001, 25(6): 665-672. |
| [10] | 徐勤松 施国新 杜开和. 锌胁迫下水车前叶细胞自由基过氧化损伤与超微结构变化之间关系的研究[J]. 植物学报, 2001, 18(05): 597-604. |
| [11] | 张志权, 束文圣, 蓝崇钰, 黄铭洪. 引入土壤种子库对铅锌尾矿废弃地植被恢复的作用[J]. 植物生态学报, 2000, 24(5): 601-607. |
| [12] | 卢善发 宋艳茹. 维管组织分化的分子生物学研究[J]. 植物学报, 1999, 16(03): 219-227. |
| [13] | 吴兆明 王玉琦 孙景信 陈红民. 小麦种子含锌量在幼苗中的分配与对缺锌敏感性的关系[J]. 植物学报, 1998, 15(04): 51-54. |
| [14] | 陈铭, 谭见安, 尹崇仁, 朱理徽. 北京石灰性草甸土冬小麦营养元素生物循环的特点与锰、锌肥效应[J]. 植物生态学报, 1995, 19(1): 64-71. |
| [15] | 葛滢, 李建东, 常杰. 东北羊草草地锰、铜、锌含量特征的研究[J]. 植物生态学报, 1994, 18(4): 338-346. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||