

Chinese Bulletin of Botany ›› 2017, Vol. 52 ›› Issue (5): 568-578.DOI: 10.11983/CBB16225 cstr: 32102.14.CBB16225
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Li Ma1, Wancang Sun1,*( ), Jinhai Yuan1, Zigang Liu1, Junyan Wu1, Yan Fang1, Yaozhao Xu2, Yuanyuan Pu1, Jing Bai1, Xiaoyun Dong1, Huili He1
), Jinhai Yuan1, Zigang Liu1, Junyan Wu1, Yan Fang1, Yaozhao Xu2, Yuanyuan Pu1, Jing Bai1, Xiaoyun Dong1, Huili He1
Received:2016-11-23
Accepted:2017-03-24
Online:2017-09-01
Published:2017-07-10
Contact:
Wancang Sun
Li Ma, Wancang Sun, Jinhai Yuan, Zigang Liu, Junyan Wu, Yan Fang, Yaozhao Xu, Yuanyuan Pu, Jing Bai, Xiaoyun Dong, Huili He. Expression Analysis of β-1,3-Glucanase Gene from Winter Brassica rapa Under Low Temperature Stress[J]. Chinese Bulletin of Botany, 2017, 52(5): 568-578.
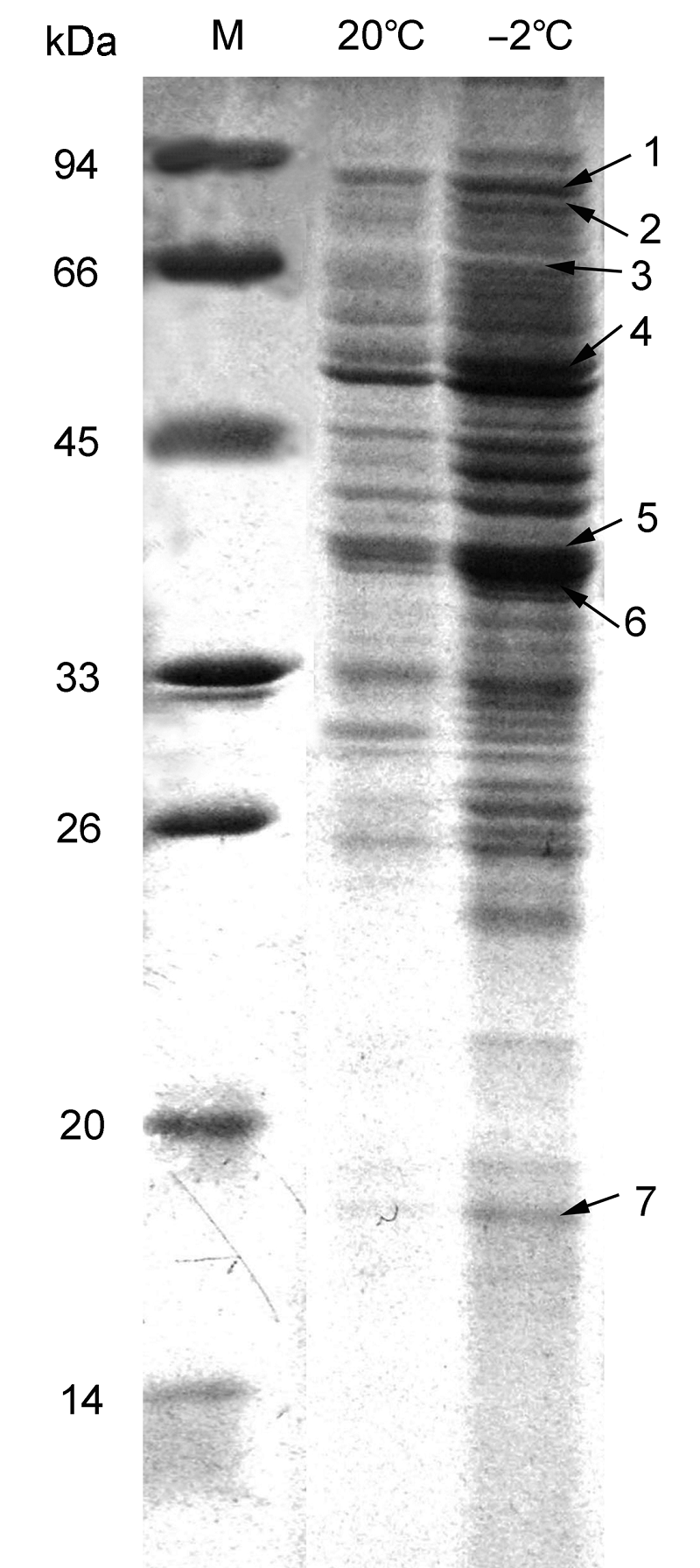
Figure 1 Apoplast protein map of Brassica rapa cv. ‘Long- you6’ leavesM: Protein marker; 1: Subtilisin-like protease; 2: Chloroplast heat shock protein 70-1; 3: Myrosinase; 4: S-adenosyl-L- homocystein hydrolase; 5: Basic glucanase; 6: β-1,3-gluca- nase; 7: Pathogenesis- related protein-1
| Spot No. | NCBI accession No. | Protein name | Organism | Score | Sequence coverage (%) |
|---|---|---|---|---|---|
| 1 | gi|757534 | Subtilisin-like protease | Arabidopsis thaliana | 146 | 3 |
| 2 | gi|166919370 | Chloroplast heat shock protein 70-1 | Ipomoea nil | 291 | 6 |
| 3 | gi|11034734 | Myrosinase | Raphanus sativus | 98 | 6 |
| 4 | gi|32967699 | S-adenosyl-L-homocystein hydrolase | A. thaliana | 290 | 29 |
| 5 | gi|118763538 | Basic glucanase | Brassica juncea | 315 | 53 |
| 6 | gi|62361691 | β-1,3-glucanase | B. rapa subsp. | 348 | 48 |
| 7 | gi|722274 | Pathogenesis-related protein-1 | B. juncea | 78 | 41 |
Table 1 Mass spectrometry results of apoplast proteins in Brassica rapa cv. ‘Longyou6’ leaves
| Spot No. | NCBI accession No. | Protein name | Organism | Score | Sequence coverage (%) |
|---|---|---|---|---|---|
| 1 | gi|757534 | Subtilisin-like protease | Arabidopsis thaliana | 146 | 3 |
| 2 | gi|166919370 | Chloroplast heat shock protein 70-1 | Ipomoea nil | 291 | 6 |
| 3 | gi|11034734 | Myrosinase | Raphanus sativus | 98 | 6 |
| 4 | gi|32967699 | S-adenosyl-L-homocystein hydrolase | A. thaliana | 290 | 29 |
| 5 | gi|118763538 | Basic glucanase | Brassica juncea | 315 | 53 |
| 6 | gi|62361691 | β-1,3-glucanase | B. rapa subsp. | 348 | 48 |
| 7 | gi|722274 | Pathogenesis-related protein-1 | B. juncea | 78 | 41 |

Figure 3 Nucleotide sequence and deduced amino acid sequence of β-1,3-glucanase cDNAAn upstream in-flame start codon ATG is boxed; * indicates the positions of termination codon TAA.
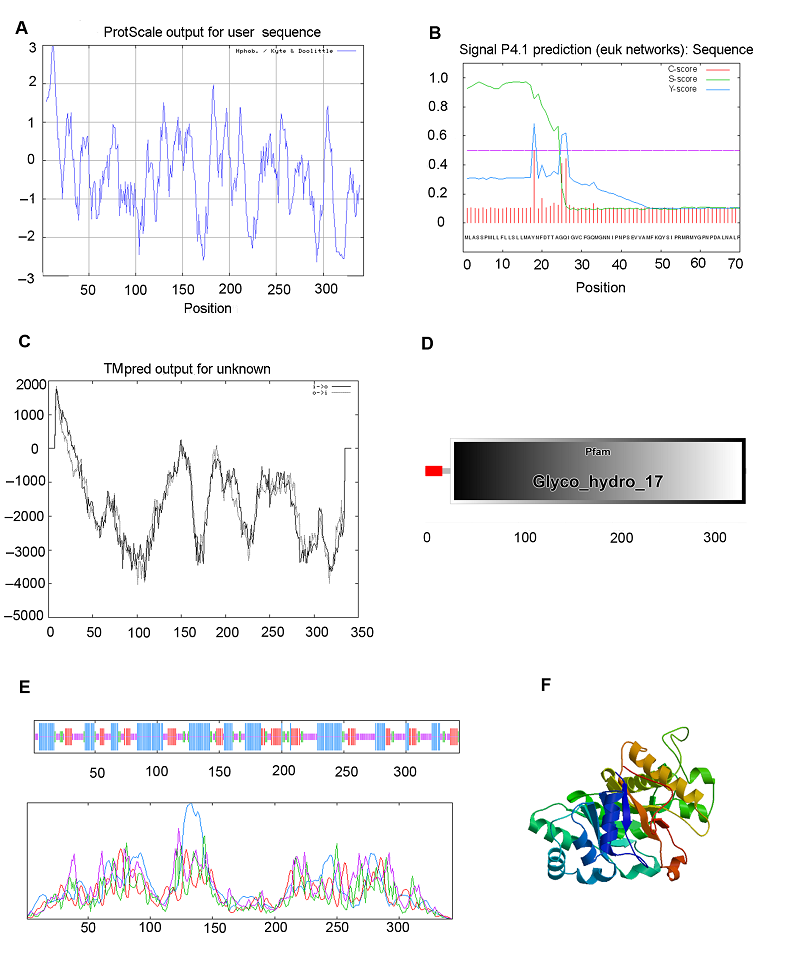
Figure 4 Bioinformatic analysis of β-1,3-glucanase protein in Brassica rapa cv. ‘Longyou6’(A) Predicted protein hydrophobicity by ProtScale; (B) Predicted signal peptide by Signal P4; (C) Transmembrane helical segments predicted by TMpred; (D) Predication of domains; (E) Predicted secondary structure; (F) Predicted 3-D structure
| Cruciferous plants | No. of amino acids | Molecular weight (kDa) | Theoretical isoelectric point | Protein instability index | Grand average of hydropathicity | Composition of amino acid | The main amino acid (%) |
|---|---|---|---|---|---|---|---|
| Longyou6 | 343 | 38.102 | 6.63 | 39.58 | -0.334 | 20 | Asn (9.6), Leu (8.7) |
| Tianyou4 | 343 | 38.130 | 7.68 | 39.02 | -0.337 | 20 | Asn (9.6), Leu (8.7) |
| Brassica rapa | 342 | 38.180 | 7.69 | 42.99 | -0.370 | 20 | Asn (9.6), Leu (8.8) |
| B. rapa subsp. chinensis | 363 | 40.659 | 9.27 | 39.84 | -0.335 | 20 | Asn (8.8), Leu (8.3) |
| B. napus | 363 | 40.599 | 9.22 | 39.96 | -0.325 | 20 | Asn (9.1), Leu (8.3) |
| B. oleracea | 351 | 38.925 | 6.85 | 40.48 | -0.304 | 20 | Asn (8.8), Ser (8.5) |
| B. oleracea var. oleracea | 365 | 41.075 | 9.27 | 37.84 | -0.390 | 20 | Asn (8.8), Leu (7.7) |
| B. juncea | 346 | 38.087 | 9.13 | 38.03 | -0.188 | 20 | Gly (9.5), Leu (9.0) |
| Raphanus sativus | 345 | 38.243 | 6.20 | 40.33 | -0.326 | 20 | Leu (10.1), Asn (8.4) |
| Arabidopsis thaliana | 355 | 39.380 | 5.47 | 47.15 | -0.318 | 20 | Ser (9.3), Leu (8.7) |
Table 2 Predicted physicochemical properties of β-1,3-glucanase gene encoding proteins in cruciferae plants
| Cruciferous plants | No. of amino acids | Molecular weight (kDa) | Theoretical isoelectric point | Protein instability index | Grand average of hydropathicity | Composition of amino acid | The main amino acid (%) |
|---|---|---|---|---|---|---|---|
| Longyou6 | 343 | 38.102 | 6.63 | 39.58 | -0.334 | 20 | Asn (9.6), Leu (8.7) |
| Tianyou4 | 343 | 38.130 | 7.68 | 39.02 | -0.337 | 20 | Asn (9.6), Leu (8.7) |
| Brassica rapa | 342 | 38.180 | 7.69 | 42.99 | -0.370 | 20 | Asn (9.6), Leu (8.8) |
| B. rapa subsp. chinensis | 363 | 40.659 | 9.27 | 39.84 | -0.335 | 20 | Asn (8.8), Leu (8.3) |
| B. napus | 363 | 40.599 | 9.22 | 39.96 | -0.325 | 20 | Asn (9.1), Leu (8.3) |
| B. oleracea | 351 | 38.925 | 6.85 | 40.48 | -0.304 | 20 | Asn (8.8), Ser (8.5) |
| B. oleracea var. oleracea | 365 | 41.075 | 9.27 | 37.84 | -0.390 | 20 | Asn (8.8), Leu (7.7) |
| B. juncea | 346 | 38.087 | 9.13 | 38.03 | -0.188 | 20 | Gly (9.5), Leu (9.0) |
| Raphanus sativus | 345 | 38.243 | 6.20 | 40.33 | -0.326 | 20 | Leu (10.1), Asn (8.4) |
| Arabidopsis thaliana | 355 | 39.380 | 5.47 | 47.15 | -0.318 | 20 | Ser (9.3), Leu (8.7) |
| [1] |
陈芳兰, 林玉玲, 陈裕坤, 冯新, 张梓浩, 赖钟雄 (2015). 三明野生蕉β-1,3-葡聚糖酶基因克隆及其低温下SA处理后的表达分析. 西北植物学报 35, 1709-1721.
DOI URL |
| [2] |
程红梅, 简桂良, 倪万潮, 杨红华, 王志兴, 孙文姬, 张保龙, 王晓峰, 马存, 贾士荣 (2005). 转几丁质酶和β-1,3-葡聚糖酶基因提高棉花对枯萎病和黄萎病的抗性. 中国农业科学 38, 1160-1166.
DOI URL |
| [3] |
邓文星, 张映 (2007). 实时荧光定量PCR技术综述. 生物技术通报(5), 93-95, 103.
DOI URL |
| [4] | 高玉龙, 郭旺珍, 王磊, 张天真 (2007). 一个棉花β-1,3-葡聚糖酶基因全长cDNA的克隆与特征分析. 作物学报 33, 1310-1315. |
| [5] | 龚束芳, 杨涛, 车代弟 (2010). 抗冻蛋白溶液中冰晶生长行为的研究. 上海交通大学学报(农业科学版) 28, 265-268, 279. |
| [6] | 郭尧君 (2005). 蛋白质电泳实验技术(第2版). 北京: 科学出版社. pp. 123-156. |
| [7] | 何江峰, 韩冰, 郭慧琴, 张占雄, 赵雅丽 (2007). 燕麦β-1,3-葡聚糖酶II基因3′端cDNA的克隆及分析. 生物技术 17(1), 5-8. |
| [8] | 何江峰, 韩冰, 赵宏鑫 (2006). 植物β-1,3-葡聚糖酶的研究进展. 内蒙古农业科技 5, 21-25. |
| [9] |
蒋选利, 李振岐, 康振生 (2005). β-1,3-葡聚糖酶与植物的抗病性. 西北农业学报 14, 135-139.
DOI URL |
| [10] |
蓝海燕, 王长海, 张丽华, 刘桂珍, 王岚兰, 陈正华, 田颖川 (2000). 导入β-1,3-葡聚糖酶及几丁质酶基因的转基因可育油菜及其抗菌核病的研究. 生物工程学报 16, 142-146.
DOI URL |
| [11] |
林植芳, 刘楠 (2012). 活性氧调控植物生长发育的研究进展. 植物学报 47, 74-86.
DOI URL |
| [12] |
马文月 (2004). 植物冷害和抗冷性的研究进展. 安徽农业科学 32, 1003-1006.
DOI URL |
| [13] |
欧阳波, 李汉霞, 叶志彪 (2002). 植物β-1,3-葡聚糖酶及其基因. 中国生物工程杂志 22, 18-23.
DOI URL |
| [14] |
孙万仓, 马卫国, 雷建民, 刘秦, 杨仁义, 武军艳, 王学芳, 叶剑, 曾军, 张亚宏, 康艳丽, 郭秀娟, 魏文惠, 杨杰, 蒲媛媛, 曾潮武, 刘红霞 (2007). 冬油菜在西北旱寒区的适应性和北移的可行性研究. 中国农业科学 40, 2716-2726.
DOI URL |
| [15] | 孙万仓, 刘自刚, 周冬梅, 张仁陟 (2016). 北方冬油菜北移与区划. 北京: 科学出版社. pp. 45-47. |
| [16] |
杨刚, 史鹏辉, 孙万仓, 刘自刚, 曾秀存, 武军艳, 方彦, 李学才, 陈奇, 刘林波, 杨建胜, 方园, 张娟 (2016). 白菜型冬油菜质外体抗冻蛋白研究. 中国生态农业学报 24, 210-217.
DOI URL |
| [17] | 翟国会, 阮小蕾, 吴丽婷, 谭小勇, 李华平 (2011). 指天蕉β-1,3-葡聚糖酶基因全长cDNA的克隆及序列分析. 中国农业科学 44, 3134-3141. |
| [18] |
张妙霞 (2010). 野生香蕉(Musa spp., AB group)抗寒相关基因的克隆与表达分析. 博士论文. 福州: 福建农林大学. pp. 134-140.
DOI URL |
| [19] | 赵娟, 兰海燕 (2011). 拟南芥β-1,3-葡聚糖酶基因(BG1)在不同组织及非生物胁迫下的表达研究. 新疆农业科学 48, 712-718. |
| [20] |
周丽英, 杨丽涛, 郑坚瑜 (2001). 植物抗寒冻基因工程研究进展. 植物学通报 18, 325-331.
DOI URL |
| [21] |
Beffa R, Meins Jr FM (1996). Pathogenesis-related function of plant β-1,3-glucanae investigated by antisense transformation—a review.Gene 179, 97-103.
DOI URL PMID |
| [22] |
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical Biochem 72, 248-254.
DOI URL |
| [23] |
Griffith M, Ala P, Yang DSC, Hon WC, Moffatt BA (1992). Antifreeze protein produced endogenously in winter rye leaves.Plant Physiol 100, 593-596.
DOI URL |
| [24] |
Hon WC, Griffith M, Chong P, Yang DSC (1994). Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves.Plant Physiol 104, 971-980.
DOI URL PMID |
| [25] |
Masoud SA, Zhu Q, Lamb C, Dixon RA (1996). Constitutive expression of an inducible β-1,3-glucanase in alfalfa reduces disease severity caused by the oomycete patho- gen Phytophthora megasperma f. spmedicaginis, but does not reduce disease severity of chitin-containing fungi.Transgenic Res 5, 313-323.
DOI URL |
| [26] |
Mezabasso L, Alberdi M, Raynal M, Ferrerocadinanos ML, Delseny M (1986). Changes in protein synthesis in rapeseed (Brassica napus) seedlings during a low temperature treatment.Plant Physiol 82, 733-738.
DOI URL PMID |
| [27] |
Nakamura Y, Sawada H, Kobayashi S, Nakajima I, Yoshikawa M (1999). Expression of soybean β-1,3-endo- glucanase cDNA and effect on disease tolerance in kiwifruit plants.Plant Cell Rep 18, 527-532.
DOI URL |
| [28] |
Xiong LM, Zhu JK (2001). Abiotic stress signal transduction in plants: molecular and genetic perspectives.Physiol Plant 112, 152-166.
DOI URL PMID |
| [29] |
Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ (1994). Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in trans- genic tobacco.Biol Technol 12, 807-812.
DOI URL |
| [1] | Yawen Zhang, Shan Liang, Guoyun Xu, Wuxia Guo, Shulin Deng. Genome-wide Identification and Analysis of CONSTANS-like Gene Family in Nicotiana tabacum [J]. Chinese Bulletin of Botany, 2021, 56(1): 33-43. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||