

Chinese Bulletin of Botany ›› 2016, Vol. 51 ›› Issue (6): 827-840.DOI: 10.11983/CBB15212 cstr: 32102.14.CBB15212
Previous Articles Next Articles
Mingjie He1,2†, Yichen Sun2†, Xiaoyuan Cheng2, Dongxue Shi2, Diqin Li1, Yiyin Chen3, Yongkun Feng2, Lu Liu2, Tengfei Fan2, Chao Yang3, Fengqiu Cao4*, Laihua Liu1,2*
Received:2015-12-03
Accepted:2016-04-01
Online:2016-11-01
Published:2016-12-02
Contact:
Cao Fengqiu,Liu Laihua
About author:# Co-first authors
Mingjie He, Yichen Sun, Xiaoyuan Cheng, Dongxue Shi, Diqin Li, Yiyin Chen, Yongkun Feng, Lu Liu, Tengfei Fan, Chao Yang, Fengqiu Cao, Laihua Liu. Current Research Advances on Glutamate Receptors (GLRs) in Plants[J]. Chinese Bulletin of Botany, 2016, 51(6): 827-840.
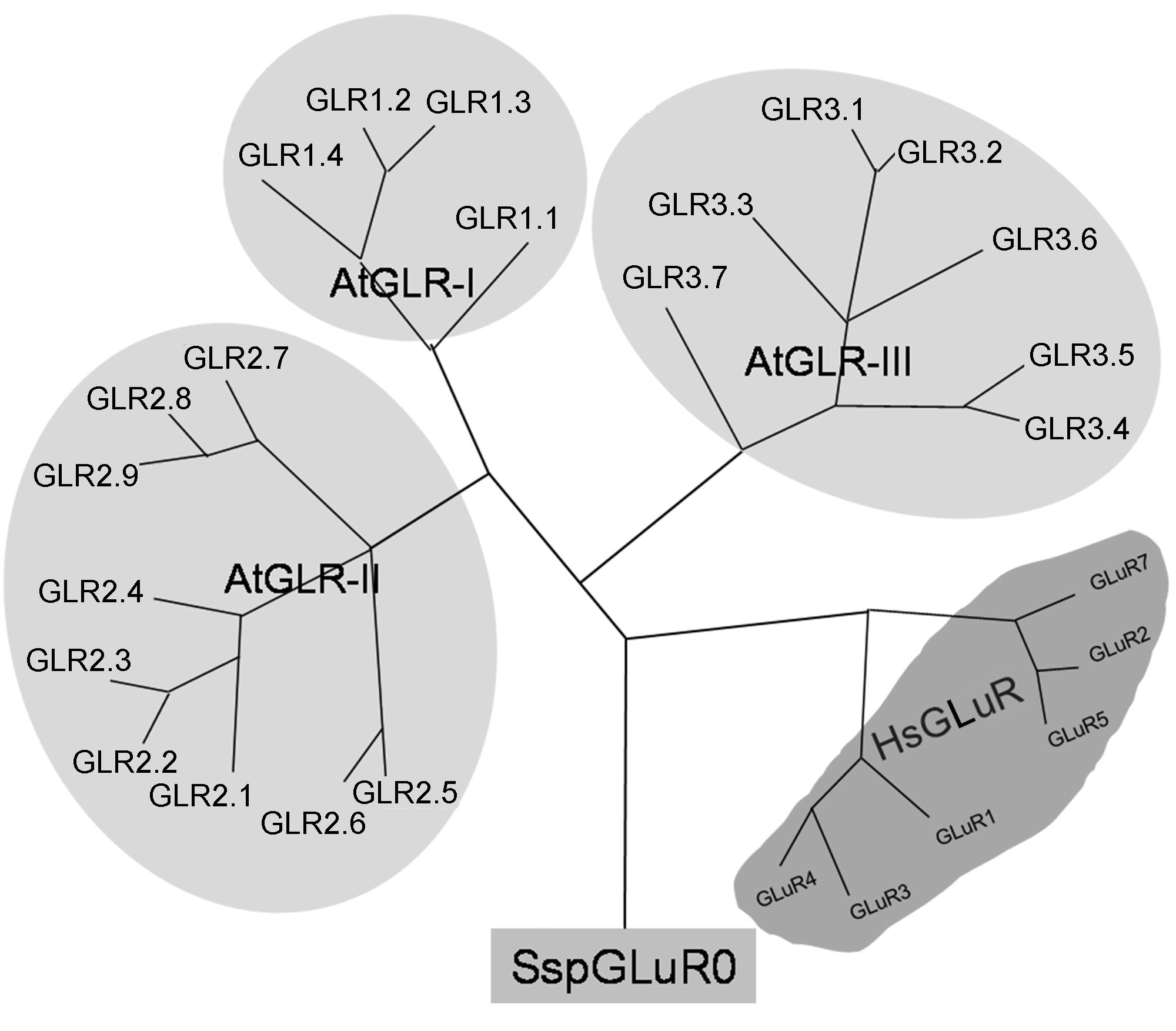
Figure 1 Phylogenetic tree of glutamate receptor homologs from Arabidopsis, human beings and bacteriumThe amino acid sequence alignment was performed using Clustal W (from Lasergene DNA*) and the phylogenetic tree was generated by parsimony analysis using PAUP* 4.10b software. At: Arabidopsis thaliana; Hs: Homo sapiens; Ssp, Synechocystis sp. (strain) PCC6803. AtGLR protein sequences were extracted from Tair, HsGLuR1-7 and SspGLuR protein sequences were obtained from NCBI database. GenBank accession number or gene models for obtaining putative full-length protein sequences are described as below: AtGLR1.1 (At3g04110), AtGLR1.2 (At5g48400), AtGLR1.3 (At5g48410), AtGLR1.4 (At3g07520), AtGLR2.1 (At5g27100), AtGLR2.2 (At2g24720), AtGLR2.3 (At2g24710), AtGLR2.4 (At4g31710), AtGLR2.5 (At5g11210), AtGLR2.6 (At5g11180), AtGLR2.7 (At2g29120), AtGLR2.8 (At2g29110), AtGLR2.9 (At2g29100), AtGLR3.1 (At2g17260), AtGLR3.2 (At4g35290), AtGLR3.3 (At1g42540), AtGLR3.4 (At1g05200), AtGLR3.5 (At2g32390), AtGLR3.6 (At3g51480), AtGLR3.7 (At2g32400); HsGLuR1 (NP_000818.1), HsGLuR2 (NP_786944.1), HsGLuR3 (NP_015564.1), HsGLuR4 (NP_000820.1), HsGLuR5 (NP_000821.1), HsGLuR6 (CAC67487.1), HsGLuR7 (NP_000822); SspGLuR0 (BAA17851.1).
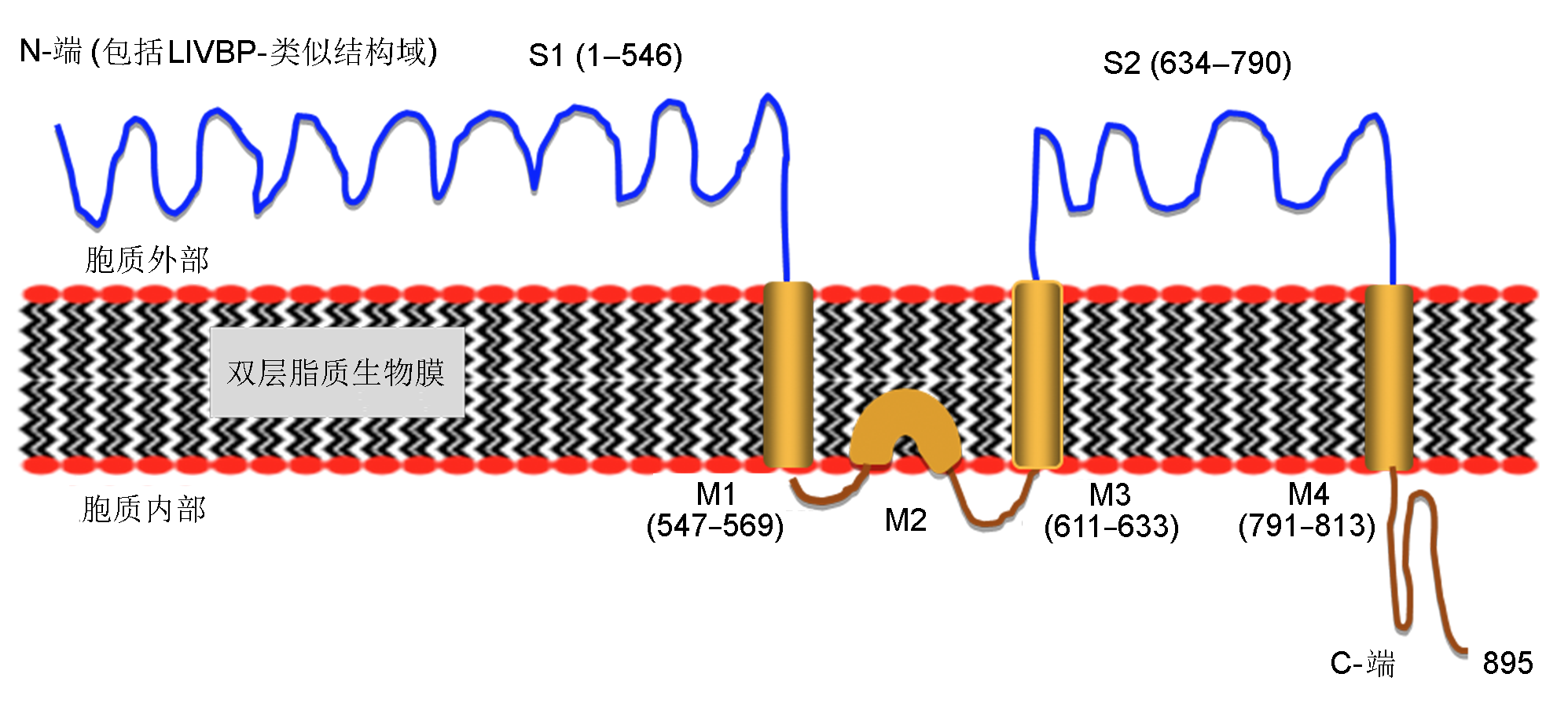
Figure 2 Putative topology of a representative glutamate receptor (GLR3.5) from ArabidopsisThe protein sequence of AtGLR3.5 was extracted from Aramemnon (http://aramemnon.uni-koeln.de/index.ep). Topological (or hydrophobicity) prediction of protein was performed using a program from a web server http://www.cbs.dtu.dk/services/TMHMM/. AtGLR3.5 is predicted to exhibit 3 trans-membrane domains (M1, M3, and M4), 1 domain (M2) imbedded in a lipid-layer, 2 domains S1 and S2 locating outside cytoplasm for ligand binding, and a C-terminal region towards the cytosol. Numbers indicate positions of amino acid residues in a peptide sequence.
| 基因名称及 登录号 | 家族 | 苗龄 | 表达位点 | 参考文献 | ||
|---|---|---|---|---|---|---|
| 器官水平 | 组织水平 | 亚细胞水平 | ||||
| AtGLR1.1 At3g04110 | I | 1周 | 根、茎、叶 | 根部皮层, 托叶着生部 | NR | Chiu et al., 2002; Roy et al., 2008 |
| 4周 | 全展叶叶沿; 根系除根尖外各组织 | NR | ||||
| 8周 | 主要是根、叶; 花、角果中很微弱 | 花、角果中检测不到GUS信号 | NR | |||
| AtGLR1.2 At5g48400 | 4周后 | 根、茎、叶、花、角果; 主要是根与角果 | 花粉管 | NR | ||
| AtGLR1.3 At5g48410 | 4周 | 根、茎、叶, 叶中很微弱 | NR | NR | ||
| 8周 | 叶和花中微弱; 主要是根与角果 | NR | NR | |||
| AtGLR1.4 At3g07520 | 4周后 | 根、茎、叶、花、角果 | NR | NR | ||
| AtGLR2.1 At5g27100 | II | 1周后 | 根、茎、叶、叶柄 | 根系除根尖外各组织; 托叶着生部位 | NR | |
| 4周后 | 根 | 根系所有组织; 花药和胚珠检测到微弱且转瞬即逝的GUS信号 | NR | |||
| AtGLR2.2 At2g24720 | 4周后 | 根 | NR | NR | ||
| AtGLR2.3 At2g24710 | 4周后 | 根 | NR | NR | ||
| AtGLR2.4 At4g31710 | 4周后 | 根、角果 | NR | NR | ||
| AtGLR2.5 At5g11210 | 4周后 | 根、茎、叶、花、角果 | NR | NR | ||
| AtGLR2.6 At5g11180 | 4周后 | 根 | NR | NR | ||
| AtGLR2.7 At2g29120 | 4周后 | 叶、角果, 根中微弱 | NR | NR | ||
| AtGLR2.8 At2g29110 | 4周后 | 根、叶柄、叶、角果 | NR | NR | Chiu et al., 2002 Roy et al., 2008 | |
| AtGLR2.9 At2g29100 | 4周 | 根、叶、茎、叶柄 | NR | NR | ||
| 8周 | 根 | NR | NR | |||
| AtGLR3.1 At2g17260 | III | 5天后 | 根、茎、叶、叶柄 | 所有维管组织; 保卫细胞 | NR | Cho et al., 2009 Kim et al., 2001; Kong et al., 2015; Meyerhoff et al., 2005 Turano et al., 2002 Teardo et al., 2011 Teardo et al., 2015 |
| 8周 | 根、茎、叶、花、角果 | 花丝和花药连接部位强烈表达 | NR | |||
| AtGLR3.2 At4g35290 | 7天后 | 根、茎、叶柄、叶 | 所有维管组织及其邻近的导管, 随着植物生长表达更强烈; 根系韧皮部细胞中表达量远高于邻近细胞, 原生韧皮部更为强烈 | NR | ||
| 8周 | 根、茎、叶、花、角果, 主要是叶与角果 | 花、茎维管组织; 花芽; 胚珠, 尤其是外皮层和生长中心 | NR | |||
| AtGLR3.3 At1g42540 | 4周后 | 根、茎、叶柄、叶、角果 | NR | |||
| AtGLR3.4 At1g05200 | 4周后 | 根、茎、叶、花、角果, 莲座叶中最强 | 叶肉组织; 维管束; 保卫细胞, 排水孔; 根内外皮层, 根毛 | 细胞质膜, 叶绿体内膜 | ||
| AtGLR3.5 At2g32390 | 0 | 种子发芽时强烈, 随发芽完成降低 | 胚胎与子叶中强烈表达 | 线粒体内膜; 叶绿体膜 | ||
| 4周后 | 根、茎、叶、花、角果 | NR | ||||
| AtGLR3.7 At2g32400 | 整个生长阶段 | 所有器官 | 所有组织 | 细胞质膜 | ||
Table 1 Overview of glutamate receptors-like receptors (AtGLRs) expression pattern in Arabidopsis thaliana
| 基因名称及 登录号 | 家族 | 苗龄 | 表达位点 | 参考文献 | ||
|---|---|---|---|---|---|---|
| 器官水平 | 组织水平 | 亚细胞水平 | ||||
| AtGLR1.1 At3g04110 | I | 1周 | 根、茎、叶 | 根部皮层, 托叶着生部 | NR | Chiu et al., 2002; Roy et al., 2008 |
| 4周 | 全展叶叶沿; 根系除根尖外各组织 | NR | ||||
| 8周 | 主要是根、叶; 花、角果中很微弱 | 花、角果中检测不到GUS信号 | NR | |||
| AtGLR1.2 At5g48400 | 4周后 | 根、茎、叶、花、角果; 主要是根与角果 | 花粉管 | NR | ||
| AtGLR1.3 At5g48410 | 4周 | 根、茎、叶, 叶中很微弱 | NR | NR | ||
| 8周 | 叶和花中微弱; 主要是根与角果 | NR | NR | |||
| AtGLR1.4 At3g07520 | 4周后 | 根、茎、叶、花、角果 | NR | NR | ||
| AtGLR2.1 At5g27100 | II | 1周后 | 根、茎、叶、叶柄 | 根系除根尖外各组织; 托叶着生部位 | NR | |
| 4周后 | 根 | 根系所有组织; 花药和胚珠检测到微弱且转瞬即逝的GUS信号 | NR | |||
| AtGLR2.2 At2g24720 | 4周后 | 根 | NR | NR | ||
| AtGLR2.3 At2g24710 | 4周后 | 根 | NR | NR | ||
| AtGLR2.4 At4g31710 | 4周后 | 根、角果 | NR | NR | ||
| AtGLR2.5 At5g11210 | 4周后 | 根、茎、叶、花、角果 | NR | NR | ||
| AtGLR2.6 At5g11180 | 4周后 | 根 | NR | NR | ||
| AtGLR2.7 At2g29120 | 4周后 | 叶、角果, 根中微弱 | NR | NR | ||
| AtGLR2.8 At2g29110 | 4周后 | 根、叶柄、叶、角果 | NR | NR | Chiu et al., 2002 Roy et al., 2008 | |
| AtGLR2.9 At2g29100 | 4周 | 根、叶、茎、叶柄 | NR | NR | ||
| 8周 | 根 | NR | NR | |||
| AtGLR3.1 At2g17260 | III | 5天后 | 根、茎、叶、叶柄 | 所有维管组织; 保卫细胞 | NR | Cho et al., 2009 Kim et al., 2001; Kong et al., 2015; Meyerhoff et al., 2005 Turano et al., 2002 Teardo et al., 2011 Teardo et al., 2015 |
| 8周 | 根、茎、叶、花、角果 | 花丝和花药连接部位强烈表达 | NR | |||
| AtGLR3.2 At4g35290 | 7天后 | 根、茎、叶柄、叶 | 所有维管组织及其邻近的导管, 随着植物生长表达更强烈; 根系韧皮部细胞中表达量远高于邻近细胞, 原生韧皮部更为强烈 | NR | ||
| 8周 | 根、茎、叶、花、角果, 主要是叶与角果 | 花、茎维管组织; 花芽; 胚珠, 尤其是外皮层和生长中心 | NR | |||
| AtGLR3.3 At1g42540 | 4周后 | 根、茎、叶柄、叶、角果 | NR | |||
| AtGLR3.4 At1g05200 | 4周后 | 根、茎、叶、花、角果, 莲座叶中最强 | 叶肉组织; 维管束; 保卫细胞, 排水孔; 根内外皮层, 根毛 | 细胞质膜, 叶绿体内膜 | ||
| AtGLR3.5 At2g32390 | 0 | 种子发芽时强烈, 随发芽完成降低 | 胚胎与子叶中强烈表达 | 线粒体内膜; 叶绿体膜 | ||
| 4周后 | 根、茎、叶、花、角果 | NR | ||||
| AtGLR3.7 At2g32400 | 整个生长阶段 | 所有器官 | 所有组织 | 细胞质膜 | ||
| [1] | Aouini A, Matsukura C, Ezura H, Asamizu E (2012). Characterization of 13 glutamate receptor-like genes encoded in the tomato genome by structure phylogeny and expression profiles.Gene 493, 36-43. |
| [2] | Ayalon G, Segev E, Elgavish S, Stern-Bach Y (2005). Two regions in the N-terminal domain of ionotropic glutamate receptor 3 form the subunit oligomerization interfaces that control subtype-specific receptor assembly.J Biol Chem 280, 15053-15060. |
| [3] | Ayalon G, Stern-Bach Y (2001). Functional assembly of AMPA and kainate receptors is mediated by several discrete protein-protein interactions.Neuron 31, 103-113. |
| [4] | Brenner ED, Martinez-Barboza N, Clark AP, Liang QS, Stevenson DW, Coruzzi GM (2000). Arabidopsis mutants resistant to S(+)-β-methyl-α, β-diaminopropionic acid, a cycad-derived glutamate receptor agonist.Plant Physiol 124, 1615-1624. |
| [5] | Chávez AE, Singer JH, Diamond JS (2006). Fast neuro- transmitter release triggered by Ca2+ influx through AMPA- type glutamate receptors.Nature 443, 705-708. |
| [6] | Chen GQ, Cui CH, Mayer ML, Gouaux E (1999). Functional characterization of a potassium-selective prokaryotic glu- tamate receptor.Nature 402, 817-821. |
| [7] | Chiu JC, Brenner ED, Desalle R, Nitabach MN, Holmes TC, Coruzzi GM (2002). Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana.Mol Biol Evol 19, 1066-1082. |
| [8] | Chiu JC, Desalle R, Lam HM, Meisel L, Coruzzi G (1999). Molecular evolution of glutamate receptors: a primitive signaling mechanism that existed before plants and animals diverged.Mol Biol Evol 16, 826-838. |
| [9] | Cho D, Kim SA, Murata Y, Lee S, Jae SK, Nam HG, Kwak JM (2009). De-regulated expression of the plant gluta- mate receptor homolog AtGLR3.1 impairs long-term Ca2+ programmed stomatal closure.Plant J 58, 437-449. |
| [10] | Davenport R (2002). Glutamate receptors in plants.Ann Bot 90, 549-557. |
| [11] | Dennison KL, Spalding EP (2000). Glutamate-gated calci- um fluxes in Arabidopsis.Plant Physiol 124, 1511-1514. |
| [12] | Dingledine R, Borges K, Bowie D, Traynelis SF (1999). The glutamate receptor ion channels. Pharmacol Rev 51, 7-61. |
| [13] | Dubos C, Huggins D, Grant GH, Knight MR, Campbell MM (2003). A role for glycine in the gating of plant NMDA- like receptors.Plant J 35, 800-810. |
| [14] | Dubos C, Willment J, Huggins D, Grant GH, Campbell MM (2005). Kanamycin reveals the role played by glutamate receptors in shaping plant resource allocation.Plant J 43, 348-355. |
| [15] | Forde BG, Cutler SR, Zaman N, Krysan PJ (2013). Gluta- mate signaling via a MEKK1 kinase-dependent pathway induces changes in Arabidopsis root architecture.Plant J 75, 1-10. |
| [16] | Kang J, Sohum M, Turano FJ (2004). The putative glutamate receptor 1.1 (AtGLR1.1) in Arabidopsis thaliana regulates abscisic acid biosynthesis and signaling to control development and water loss.Plant Cell Physiol 45, 1380-1389. |
| [17] | Kang J, Turano FJ (2003). The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana.Proc Natl Acad Sci USA 100, 6872-6877. |
| [18] | Kang S, Kim HB, Lee H, Choi JY, Heu S, Oh CJ, Kwon SI, An CS (2006). Overexpression in Arabidopsis of a plasma membrane-targeting glutamate receptor from small radish increases glutamate-mediated Ca2+ influx and delays fun- gal infection.Mol Cell 21, 418-427. |
| [19] | Kim SA, Kwak JM, Jae SK, Wang MH, Nam HG (2001). Overexpression of the AtGluR2 gene encoding an Arabi- dopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants.Plant Cell Physiol 42, 74-84. |
| [20] | Kong DD, Ju CL, Parihar A, Kim S, Cho D, Kwak JM (2015). Arabidopsis glutamate receptor homolog atglr3.5 modulates cytosolic Ca2+ level to counteract effect of abscisic acid in seed germination.Plant Physiol 167, 1630-1642. |
| [21] | Kushwaha R, Singh A, Chattopadhyay S (2008). Calmo- dulin7 plays an important role as transcriptional regulator in Arabidopsis seedling development.Plant Cell 20, 1747-1759. |
| [22] | Lacombe B (2001). The identity of plant glutamate recap- tors.Science 292, 1486-1487. |
| [23] | Lam H, Chiu J, Hsieh M, Lee M, Oliveira IC, Shin M, Coruzzi G (1998). Glutamate-receptor genes in plants.Nature 396, 125-126. |
| [24] | Li HJ, Yang WC (2012). Emerging role of ER quality control in plant cell signal perception.Protein Cell 3, 10-16. |
| [25] | Li J, Zhu SH, Song XW, Shen Y, Chen HM, Yu J, Yi K, Liu YF, Karplus VJ, Wu P, Deng XW (2006). A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem.Plant Cell 18, 340-349. |
| [26] | Manzoor H, Kelloniemi J, Chiltz A, Wendehenne D, Pugin A, Poinssot B, Garcia-Brugger A (2013). Involvement of the glutamate receptor AtGLR3.3 in plant defense sig- naling and resistance to Hyaloperonospora arabidopsidis.Plant J 76, 466-480. |
| [27] | McFeeters RL, Oswald RE (2004). Emerging structural explanations of ionotropic glutamate receptor function.FASEB J 18, 428-438. |
| [28] | Meyerhoff O, Müller K, Roelfsema MR, Latz A, Lacombe B, Hedrich R, Dietrich P, Becker D (2005). AtGLR3.4, a glutamate receptor channel-like gene is sensitive to touch and cold.Planta 222, 418-427. |
| [29] | Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham MG, Liu LH, Obermeyer G, Feijó J (2011). Glutamate receptor-like genes form Ca2+ chan- nels in pollen tubes and are regulated by pistil D-serine.Science 332, 434-437. |
| [30] | Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013). GLUTAMATE RECEPTOR-LIKE ge- nes mediate leaf-to-leaf wound signaling.Nature 500, 422-441. |
| [31] | Nagata T, Iizumi S, Satoh K, Ooka H, Kawai J, Carninci P, Hayashizaki Y, Otomo Y, Murakami K, Matsubara K, Kikuchi S (2004). Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data.Mol Biol Evol 21, 1855-1870. |
| [32] | Parsons CG, Panchenko VA, Pinchenko VO, Tsyndreko AY, Krishtal OA (1966). Comparative patch-clamp stud- ies with freshly dissociated rat hippocampal and striatal neurons on the NMDA receptor antagonistic effects of amantadine and memantin.Eur J Neurosci 8, 446-454. |
| [33] | Penn AC, Williams SR, Greger IH (2008). Gating motions underlie AMPA receptor secretion from the endoplasmic reticulum.EMBO J 27, 3056-3068. |
| [34] | Price MB, Jelesko J, Okumoto S (2013). Glutamate receptor homologs in plants: functions and evolutionary origins.Front Plant Sci 3, 1-10. |
| [35] | Price MB, Kong DD, Okumoto S (2013). Inter-subunit in- teractions between glutamate-like receptors in Arabi- dopsis.Plant Signal Behav 8, e27034. |
| [36] | Qi Z, Stephens NR, Spalding EP (2006). Calcium entry me- diated by glr3.3, an Arabidopsis glutamate receptor with a broad agonist profile.Plant Physiol 142, 963-971. |
| [37] | Roy SJ, Gilliham M, Berger B, Essah PA, Cheffings C, Miller AJ, Davenport RJ, Liu LH, Skynner MJ, Davies JM, Richardson P, Leigh RA, Tester M (2008). Investi- gating glutamate receptor-like gene co-expression in Ara- bidopsis thaliana.Plant Cell Environ 31, 861-871. |
| [38] | Singh A, Kanwar P, Yadav AK, Mishra M, Jha SK, Baranwal V, Pandey A, Kappor S, Tyagi AK, Pandey GK (2014). Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice.FEBS J 281, 894-915. |
| [39] | Sivaguru M, Pike S, Gassmann W, Baskin TI (2003). Aluminum rapidly depolymerized cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor.Plant Cell Physiol 44, 667-675. |
| [40] | Sobolevsky AI, Rosconi MP, Gouaux E (2009). X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor.Nature 462, 745-756. |
| [41] | Stephens NR, Qi Z, Spalding EP (2008). Glutamate re- ceptor subtypes evidenced by differences in desensitiza- tion and dependence on the GLR3.3 and GLR3.4 genes.Plant Physiol 146, 529-538. |
| [42] | Tapken D, Anschütz U, Liu LH, Huelsken T, Seebohm G, Becker D, Hollmann M (2013). A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids.Sci Signal 6, a47. |
| [43] | Tapken D, Hollmann M (2008). Arabidopsis thaliana gluta- mate receptor ion channel function demonstrated by ion pore transplantation.J Mol Biol 383, 36-48. |
| [44] | Teardo E, Carraretto L, Bortoli SD, Costa A, Behera S, Wagner R, Schiavo FL, Formentin E, Szabo I (2015). Alternative splicing-mediated targeting of the Arabidopsis GLUTAMATE RECEPTOR3.5 to mitochondria affects or- ganelle morphology1.Plant Physiol 167, 216-227. |
| [45] | Teardo E, Formentin E, Segalla A, Giacometti GM, Marin O, Zanetti MA, Schiavo FL, Zoratti M, Szabò I (2011). Dual localization of plant glutamate receptor AtGLR3.4 to plastids and plasmamembrane.Biochim Biophys Acta 1807, 359-367. |
| [46] | Teardo E, Segalla A, Formentin E, Zanetti M, Marin O, Giacometti GM, Schiavo FL, Zoratti M, Szabò I (2010). Characterization of a plant glutamate receptor activity.Cell Physiol Biochem 26, 253-262. |
| [47] | Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan HJ, Myers SJ, Dingledine R (2010). Glutamate receptor ion chan- nels: structure, regulation, and function.Pharmacol Rev 62, 405-496. |
| [48] | Turano FJ, Muhitch MJ, Felker FC, McMahon MB (2002). The putative glutamate receptor 3.2 from Arabidopsis thaliana (AtGLR3.2) is an integral membrane peptide that accumulates in rapidly growing tissues and persists in vascular-associated tissues.Plant Sci 163, 43-51. |
| [49] | Turano FJ, Panta GR, Allard MW, Berkum PV (2001). The putative glutamate receptors from plants are related to two superfamilies of animal neurotransmitter receptors via distinct evolutionary mechanisms.Mol Biol Evol 18, 1417-1420. |
| [50] | Ulbrich MH, Isacoff EY (2008). Rules of engagement for NMDA receptor subunits.Proc Natl Acad Sci USA 105, 14163-14168. |
| [51] | Vatsa P, Chiltz A, Bourque S, Wendehenne D, Garcia- Brugger A, Pugin A (2011). Involvement of putative glutamate receptors in plant defence signaling and NO production.Biochimie 93, 2095-2101. |
| [52] | Vincill ED, Bieck AM, Spalding EP (2012). Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors.Plant Physiol 159, 40-46. |
| [53] | Vincill ED, Clarin AE, Molenda JN, Spalding EP (2013). Interacting glutamate receptor-like proteins in phloem re- gulate lateral root initiation in Arabidopsis.Plant Cell 25, 1304-1313. |
| [54] | Walch-Liu P, Forde BG (2008). Nitrate signaling mediated by the NRT1.1 nitrate transporter antagonises L-gluta- mate-induced changes in root architecture.Plant J 54, 820-828. |
| [55] | Walch-Liu P,Liu LH, Remans T, Tester M, Forde BG (2006). Evidence that L-glutamate can act as an exoge- nous signal to modulate root growth and branching in Arabidopsis thaliana.Plant Cell Physiol 47, 1045-1057. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||