Research Progress on Physiological Functions of Suberin lamellae in Water and Solutes Transport
- Biao Zhang ,
- Jian Wu ,
- Yang Zhang ,
- Xiaowei Dong ,
- Shuo Han ,
- Xin Gao ,
- Congwu Du ,
- Huiying Li ,
- Xuefa Chong ,
- Yingying Zhu ,
- Haiwei Liu
- 1Institute of Tobacco Research of Chinese Academy of Agricultural Sciences/Key Laboratory of Tobacco Biology and Processing, Ministry of Agriculture and Rural Affairs, Qingdao 266101, China
2Graduate School of Chinese Academy of Agricultural Sciences, Beijing 100081, China
3Shandong Linyi Tobacco Co., Ltd., Linyi 276000, China
4China Tobacco Shandong Industrial Co., Ltd., Jinan 250000, China
Received date: 2022-08-31
Accepted date: 2023-01-10
Online published: 2023-01-13
Abstract
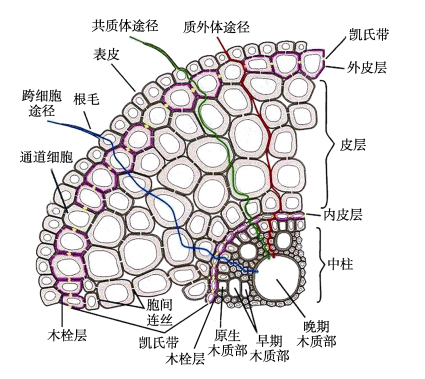
Plant roots can acquire water and nutrients selectively from soil and transport them upwards to the aerial parts for plant growth and development. These functions are closely related to their anatomical structures. The radial transport of water and solutes absorbed by roots mainly includes three different pathways, namely, the apoplastic pathway, the symplastic pathway and the transcellular pathway. The endodermis is the innermost cell layer that surrounds the central vasculature. For a long time, endodermal differentiation formed the Casparian strips has been considered to play a decisive role in blocking water and solutes transport through the apoplastic pathway. However, in recent years, it has been found that suberin lamellae formed by endodermal differentiation plays no less important role in the radial transport of water and solutes than Casparian strips, and even that suberization is the second life of an endodermal cell. In this paper, we reviewed the latest research progress on the physiological function of suberin lamellae in water and solutes transport in recent years, and discussed the relationship between suberin lamellae and drought, salt, nutrient and heavy metal stress of crops, in order to provide a reference for the theory and practice of endodermal plasticity in the regulation of plant physiological function.
Key words: root; endodermis; suberin lamellae; water and solutes; physiological functions
Cite this article
Biao Zhang , Jian Wu , Yang Zhang , Xiaowei Dong , Shuo Han , Xin Gao , Congwu Du , Huiying Li , Xuefa Chong , Yingying Zhu , Haiwei Liu . Research Progress on Physiological Functions of Suberin lamellae in Water and Solutes Transport[J]. Chinese Bulletin of Botany, 2023 , 58(6) : 1008 -1018 . DOI: 10.11983/CBB22208
References
| [1] | 韩雪源, 茅林春 (2017). 木栓质组成成分、组织化学特性及其生物合成研究进展. 植物学报 52, 358-374. |
| [2] | 刘鑫, 王沛, 周青平 (2021). 植物根系质外体屏障研究进展. 植物学报 56, 761-773. |
| [3] | 王璐瑶, 陈謇, 赵守清, 闫慧莉, 许文秀, 刘若溪, 麻密, 虞轶俊, 何振艳 (2022). 水稻镉积累特性的生理和分子机制研究概述. 植物学报 57, 236-249. |
| [4] | 许德蓉, 孙超, 毕真真, 秦天元, 王一好, 王小东, 梁文君, 李鹏程, 张俊莲, 白江平 (2021). 作物根构型相关基因研究进展及其在马铃薯抗旱种质创新中的应用展望. 植物生理学报 57, 1007-1022. |
| [5] | 杨蔚, 罗小燕, 王文强, 严琳玲, 杨虎彪, 董荣书, 徐彬, 胡国富, 刘一明, 刘国道 (2022). 细胞壁在植物抗盐胁迫中的作用. 植物生理学报 58, 501-510. |
| [6] | 张妍, 葛颜锐, 赵冉, 胡云涛, 陈羽, 郭亚玉, 林金星, 李瑞丽 (2022). 木栓质的结构组分、生物合成及其功能的研究进展. 科学通报 67, 822-833. |
| [7] | Alassimone J, Naseer S, Geldner N (2010). A develop-mental framework for endodermal differentiation and polarity. Proc Natl Acad Sci USA 107, 5214-5219. |
| [8] | Andersen TG, Barberon M, Geldner N (2015). Suberiza-tion—the second life of an endodermal cell. Curr Opin Plant Biol 28, 9-15. |
| [9] | Andersen TG, Naseer S, Ursache R, Wybouw B, Smet W, De Rybel B, Vermeer JEM, Geldner N (2018). Diffusible repression of cytokinin signaling produces endodermal symmetry and passage cells. Nature 555, 529-533. |
| [10] | Armand T, Cullen M, Boiziot F, Li LY, Fricke W (2019). Cortex cell hydraulic conductivity, endodermal apoplastic barriers and root hydraulics change in barley (Hordeum vulgare L.) in response to a low supply of N and P. Ann Bot 124, 1091-1107. |
| [11] | Barberon M (2017). The endodermis as a checkpoint for nutrients. New Phytol 213, 1604-1610. |
| [12] | Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, Geldner N (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447-459. |
| [13] | Barbosa ICR, Rojas-Murcia N, Geldner N (2019). The Casparian strip—one ring to bring cell biology to lignification? Curr Opin Biotechnol 56, 121-129. |
| [14] | Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5, e1000492. |
| [15] | Chen A, Liu T, Deng Y, Xiao R, Zhang T, Wang Y, Yang Y, Lakshmanan P, Shi X, Zhang F, Chen X (2023). Nitrate-dependent suberization regulates cadmium uptake and accumulation in maize. Sci Total Environ 878, 162848. |
| [16] | Chen AL, Husted S, Salt DE, Schjoerring JK, Persson DP (2019). The intensity of manganese deficiency strongly affects root endodermal suberization and ion homeostatsis. Plant Physiol 181, 729-742. |
| [17] | Chen HF, Zhang Q, Lv W, Yu XY, Zhang ZH (2022). Ethylene positively regulates Cd tolerance via reactive oxygen species scavenging and apoplastic transport barrier formation in rice. Environ Pollut 302, 119063. |
| [18] | Cheng H, Inyang A, Li CD, Fei J, Zhou YW, Wang YS (2020). Salt tolerance and exclusion in the mangrove plant Avicennia marina in relation to root apoplastic barriers. Ecotoxicology 29, 676-683. |
| [19] | Cohen H, Fedyuk V, Wang CH, Wu S, Aharoni A (2020). SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J 102, 431-447. |
| [20] | Cortaga CQ, Sebidos RF (2019). Drought-induced modify-cations on the outer part of the root (OPR) and root endodermis of selected rice genotypes. J Crop Sci Biotechnol 22, 131-138. |
| [21] | Cui B, Liu RR, Flowers TJ, Song J (2021). Casparian bands and suberin lamellae: key targets for breeding salt tolerant crops? Environ Exp Bot 191, 104600. |
| [22] | de Silva NDG, Murmu J, Chabot D, Hubbard K, Ryser P, Molina I, Rowland O (2021). Root suberin plays important roles in reducing water loss and sodium uptake in Arabidopsis thaliana. Metabolites 11, 735. |
| [23] | Deeken R, Saupe S, Klinkenberg J, Riedel M, Leide J, Hedrich R, Mueller TD (2016). The nonspecific lipid transfer protein AtLtpI-4 is involved in suberin formation of Arabidopsis thaliana crown galls. Plant Physiol 172, 1911-1927. |
| [24] | Doblas VG, Geldner N, Barberon M (2017a). The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 39, 136-143. |
| [25] | Doblas VG, Smakowska-Luzan E, Fujita S, Alassimone J, Barberon M, Madalinski M, Belkhadir Y, Geldner N (2017b). Root diffusion barrier control by a vasculaturederived peptide binding to the SGN3 receptor. Science 355, 280-284. |
| [26] | Emonet A, Zhou F, Vacheron J, Heiman CM, Tendon VD, Ma KW, Schulze-Lefert P, Keel C, Geldner N (2021). Spatially restricted immune responses are required for maintaining root meristematic activity upon detection of bacteria. Curr Biol 31, 1012-1028. |
| [27] | Fr?schel C, Komorek J, Attard A, Marsell A, Lopez-Arboleda WA, Le Berre J, Wolf E, Geldner N, Waller F, Korte A, Dr?ge-Laser W (2021). Plant roots employ cell- layer-specific programs to respond to pathogenic and beneficial microbes. Cell Host Microbe 29, 299-310. |
| [28] | Geldner N (2013). The endodermis. Annu Rev Plant Biol 64, 531-558. |
| [29] | Gou MY, Hou GC, Yang HJ, Zhang XB, Cai YH, Kai GY, Liu CJ (2017). The MYB107 transcription factor positively regulates suberin biosynthesis. Plant Physiol 173, 1045-1058. |
| [30] | Grünhofer P, Guo YY, Li RL, Lin JX, Schreiber L (2021). Hydroponic cultivation conditions allowing the reproducible investigation of poplar root suberization and water transport. Plant Methods 17, 129. |
| [31] | Gupta A, Rico-Medina A, Ca?o-Delgado AI (2020). The physiology of plant responses to drought. Science 368, 266-269. |
| [32] | Holbein J, Shen DF, Andersen TG (2021). The endodermal passage cell—just another brick in the wall? New Phytol 230, 1321-1328. |
| [33] | Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE (2013). Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based casparian strip in the root. Proc Natl Acad Sci USA 110, 14498-14503. |
| [34] | Hsu YF, Yan JW, Song Y, Zheng M (2021). Sarracenia purpurea glycerol-3-phosphate acyltransferase 5 confers plant tolerance to high humidity in Arabidopsis thaliana. Physiol Plant 173, 1221-1229. |
| [35] | Huang L, Li WC, Tam NFY, Ye ZH (2019). Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J Environ Sci 75, 296-306. |
| [36] | Kim G, Ryu H, Sung J (2022). Hormonal crosstalk and root suberization for drought stress tolerance in plants. Biomolecules 12, 811. |
| [37] | Kim YX, Ranathunge K, Lee S, Lee Y, Lee D, Sung J (2018). Composite transport model and water and solute transport across plant roots: an update. Front Plant Sci 9, 193. |
| [38] | Knipfer T, Danjou M, Vionne C, Fricke W (2021). Salt stress reduces root water uptake in barley (Hordeum vulgare L.) through modification of the transcellular transport path. Plant Cell Environ 44, 458-475. |
| [39] | Kreszies T, Eggels S, Kreszies V, Osthoff A, Shellakkutti N, Baldauf JA, Zeisler-Diehl VV, Hochholdinger F, Ranathunge K, Schreiber L (2020a). Seminal roots of wild and cultivated barley differentially respond to osmotic stress in gene expression, suberization, and hydraulic conductivity. Plant Cell Environ 43, 344-357. |
| [40] | Kreszies T, Kreszies V, Ly F, Thangamani PD, Shellak-kutti N, Schreiber L (2020b). Suberized transport barriers in plant roots: the effect of silicon. J Exp Bot 71, 6799-6806. |
| [41] | Kreszies T, Schreiber L, Ranathunge K (2018). Suberized transport barriers in Arabidopsis, barley and rice roots: from the model plant to crop species. J Plant Physiol 227, 75-83. |
| [42] | Kreszies T, Shellakkutti N, Osthoff A, Yu P, Baldauf JA, Zeisler-Diehl VV, Ranathunge K, Hochholdinger F, Schreiber L (2019). Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: analysis of chemical, transcriptomic and physiological responses. New Phytol 221, 180-194. |
| [43] | Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK (2009). The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sa-tiva L.). Planta 230, 119-134. |
| [44] | Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK (2011). Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J Exp Bot 62, 4215-4228. |
| [45] | Krishnamurthy P, Vishal B, Bhal A, Kumar PP (2021). WRKY9 transcription factor regulates cytochrome P450 genes CYP94B3 and CYP86B1, leading to increased root suberin and salt tolerance in Arabidopsis. Physiol Plant 172, 1673-1687. |
| [46] | Krishnamurthy P, Vishal B, Ho WJ, Lok FCJ, Lee FSM, Kumar PP (2020). Regulation of a cytochrome P450 gene CYP94B1 by WRKY33 transcription factor controls apoplastic barrier formation in roots to confer salt tolerance. Plant Physiol 184, 2199-2215. |
| [47] | Lashbrooke J, Cohen H, Levy-Samocha D, Tzfadia O, Panizel I, Zeisler V, Massalha H, Stern A, Trainotti L, Schreiber L, Costa F, Aharoni A (2016). MYB107 and MYB9 homologs regulate suberin deposition in angiosperms. Plant Cell 28, 2097-2116. |
| [48] | Lee SB, Suh MC (2018). Disruption of glycosylphosphatidy-linositol-anchored lipid transfer protein 15 affects seed coat permeability in Arabidopsis. Plant J 96, 1206-1217. |
| [49] | Lí?ka D, Martinka M, Kohanová J, Lux A (2016). Asymme-trical development of root endodermis and exodermis in reaction to abiotic stresses. Ann Bot 118, 667-674. |
| [50] | Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L (2015). Aquaporins in plants. Physiol Rev 95, 1321-1358. |
| [51] | Melino VJ, Plett DC, Bendre P, Thomsen HC, Zeisler- Diehl VV, Schreiber L, Kronzucker HJ (2021). Nitrogen depletion enhances endodermal suberization without restricting transporter-mediated root NO3- influx. J Plant Physiol 257, 153334. |
| [52] | Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012). Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA 109, 10101-10106. |
| [53] | Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JEM, Yamazaki M, Li GW, Maurel C, Takano J, Kamiya T, Salt DE, Roppolo D, Geldner N (2014). A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3, e03115. |
| [54] | Ranathunge K, Schreiber L (2011). Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J Exp Bot 62, 1961-1974. |
| [55] | Ranathunge K, Schreiber L, Bi YM, Rothstein SJ (2016). Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 243, 231-249. |
| [56] | Ranathunge K, Schreiber L, Franke R (2011). Suberin research in the genomics era—new interest for an old polymer. Plant Sci 180, 399-413. |
| [57] | Redjala T, Zelko I, Sterckeman T, Legué V, Lux A (2011). Relationship between root structure and root cadmium uptake in maize. Environ Exp Bot 71, 241-248. |
| [58] | Salas-González I, Reyt G, Flis P, Custódio V, Gopaulc-han D, Bakhoum N, Dew TP, Suresh K, Franke RB, Dangl JL, Salt DE, Castrillo G (2021). Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 371, eabd0695. |
| [59] | Shanmugarajah K, Linka N, Gr?fe K, Smits SHJ, Weber APM, Zeier J, Schmitt L (2019). ABCG1 contributes to suberin formation in Arabidopsis thaliana roots. Sci Rep 9, 11381. |
| [60] | Shiono K, Ando M, Nishiuchi S, Takahashi H, Watanabe K, Nakamura M, Matsuo Y, Yasuno N, Yamanouchi U, Fujimoto M, Takanashi H, Ranathunge K, Franke RB, Shitan N, Nishizawa NK, Takamure I, Yano M, Tsutsumi N, Schreiber L, Yazaki K, Nakazono M, Kato K (2014). RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J 80, 40-51. |
| [61] | Shukla V, Barberon M (2021). Building and breaking of a barrier: suberin plasticity and function in the endodermis. Curr Opin Plant Biol 64, 102153. |
| [62] | Shukla V, Han JP, Cléard F, Lefebvre-Legendre L, Gully K, Flis P, Berhin A, Andersen TG, Salt DE, Nawrath C, Barberon M (2021). Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transc-a)ription factors. Proc Natl Acad Sci USA 118, e21017301-18. |
| [63] | Tao Q, Jupa R, Liu YK, Luo JP, Li JX, Ková? J, Li B, Li QQ, Wu KR, Liang YC, Lux A, Wang CQ, Li TQ (2019). Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ 42, 1425-1440. |
| [64] | Tao Q, Li M, Xu Q, Ková? J, Yuan S, Li B, Li QQ, Huang R, Gao XS, Wang CQ (2022). Radial transport difference mediated by root endodermal barriers contributes to differential cadmium accumulation between japonica and indica subspecies of rice (Oryza sativa L.). J Hazard Mater 425, 128008. |
| [65] | Ursache R, De Jesus Vieira Teixeira C, Tendon VD, Gully K, De Bellis D, Schmid-Siegert E, Andersen TG, Shekhar V, Calderon S, Pradervand S, Nawrath C, Geldner N, Vermeer JEM (2021). GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat Plants 7, 353-364. |
| [66] | Vaculík M, Konlechner C, Langer I, Adlassnig W, Puschenreiter M, Lux A, Hauser MT (2012). Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environ Pollut 163, 117-126. |
| [67] | Wan JP, Wang RL, Zhang P, Sun LL, Ju Q, Huang HD, Lü SY, Tran LS, Xu J (2021). MYB70 modulates seed germination and root system development in Arabidopsis. iS-cience 24, 103228. |
| [68] | Wang P, Calvo-Polanco M, Reyt G, Barberon M, Cham-peyroux C, Santoni V, Maurel C, Franke RB, Ljung K, Novak O, Geldner N, Boursiac Y, Salt DE (2019). Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci Rep 9, 4227. |
| [69] | Wang P, Wang CM, Gao L, Cui YN, Yang HL, de Silva NDG, Ma Q, Bao AK, Flowers TJ, Rowland O, Wang SM (2020). Aliphatic suberin confers salt tolerance to Arabidopsis by limiting Na+influx, K+ efflux and water backflow. Plant Soil 448, 603-620. |
| [70] | Zhang L, Merlin I, Pascal S, Bert PF, Domergue F, Gam-betta GA (2020). Drought activates MYB41 orthologs and induces suberization of grapevine fine roots. Plant Direct 4, e00278. |