Determination of Acidic Plant Hormones by Derivative UPLC-MS
- Experimental Center of Life Sciences, College of Life Sciences, Nanjing Agricultural University, Nanjing 210095, China
Received date: 2022-01-18
Accepted date: 2022-06-23
Online published: 2022-06-23
Abstract
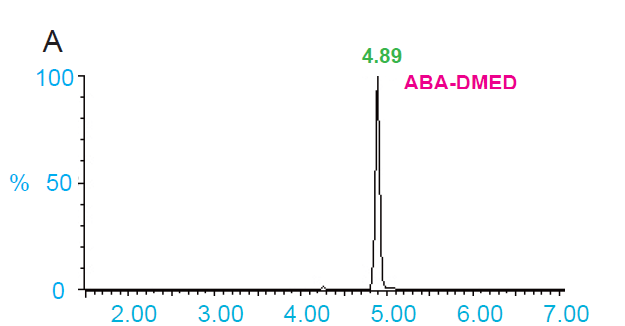
This study aims to establish a rapid and efficient method for qualitative and quantitative determination of plant acid hormones by ultra-high performance liquid chromatography/high resolution time-of-flight mass spectrometry (UPLC- TOF-MS). Several derivatization reagents, 2-bromoacetophenone (BP), 2-dimethylaminoethylamine (DMED), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) and 3-bromoactonyltrimethylammonium bromide (BTA) were selected for derivatization with abscisic acid, gibberellic acid, indoleacetic acid, jasmonic acid and salicylic acid, respectively. The effect of derivatization reagent was evaluated by comparing the response value of the derivative hormones. The plant hormone standards were diluted in gradient and reacted with the derivatization reagent, and the detection limit and quantitation limit of the derivative hormones were determined. Reagent with the best derivatization effect was reacted with the plant crude extract to test its application effect. All the four derivatized reagents could improve the MS response value of acidic hormone, among which the MS sensitivity of hormone derived by DMED is the best. Reaction with DMED was stable and reproducible. The quantitation limits of ABA-DMED, GA3-DMED, IAA-DMED, JA-DMED and SA-DMED were 0.05, 0.2, 0.1, 0.1 and 0.5 ng∙mL-1, respectively. Compared with non-derivatized hormones, the quantitative limits of ABA, GA3, IAA, JA and SA were reduced by 100, 25, 50, 50 and 10 times, respectively. So, DMED derivatization increased the mass spectrometry sensitivity of several hormones by 10-100 times. The method was applied to the determination of endogenous acidic hormones in rice, wheat and broad bean, and the sensitivity was significantly higher than that before derivation. A simple, rapid and reproducible UPLC-TOF-MS method based on derivatization was established, which greatly improved the sensitivity of the determination of acidic hormones in plants.
Cite this article
Dai Chen , Wang Jin , Lu Yaping . Determination of Acidic Plant Hormones by Derivative UPLC-MS[J]. Chinese Bulletin of Botany, 2022 , 57(4) : 500 -507 . DOI: 10.11983/CBB22017
References
| [1] | 陈鸣銮 (2013). 基于整体柱的微分离分析方法研究及其在酸性植物激素检测中的应用. 博士论文. 武汉: 武汉大学. pp. 36-59. |
| [2] | 张晨曦, 卢爱玉, 薛扬睿, 孙崟喆, 江弦, 许风国, 焦宇 (2019). 衍生化HPLC/MS法测定有机羧酸物质的研究进展. 药学研究 38, 657-662. |
| [3] | Barkawi LS, Tam YY, Tillman JA, Normanly J, Cohen JD (2010). A high-throughput method for the quantitative analysis of auxins. Nat Protoc 5, 1609-1618. |
| [4] | Barkawi LS, Tam YY, Tillman JA, Pederson B, Calio J, Al-Amier H, Emerick M, Normanly J, Cohen JD (2008). A high-throughput method for the quantitative analysis of indole-3-acetic acid and other auxins from plant tissue. Anal Biochem 372, 177-188. |
| [5] | Du FY, Ruan GH, Liu HW (2012). Erratum to: analytical methods for tracing plant hormones. Anal Bioanal Chem 404, 1615. |
| [6] | Hao YH, Zhang Z, Wang L, Liu C, Lei AW, Yuan BF, Feng YQ (2015). Stable isotope labeling assisted liquid chromatography-electrospray tandem mass spectrometry for quantitative analysis of endogenous gibberellins. Talanta 144, 341-348. |
| [7] | Hedden P (1993). Modern methods for the quantitative analysis of plant hormones. Annu Rev Plant Physiol Plant Mol Biol 44, 107-129. |
| [8] | Higashi T, Shibayama Y, Ichikawa T, Ito K, Toyo’Oka T, Shimada K, Mitamura K, Ikegawa S, Chiba H (2010). Salivary chenodeoxycholic acid and its glycine-conjugate: their determination method using LC-MS/MS and variation of their concentrations with increased saliva flow rate. Ste- roids 75, 338-345. |
| [9] | Izumi Y, Okazawa A, Bamba T, Kobayashi A, Fukusaki E (2009). Development of a method for comprehensive and quantitative analysis of plant hormones by highly sensitive nano flow liquid chromatography-electrospray ionization ion trap mass spectrometry. Anal Chim Acta 648, 215-225. |
| [10] | Li DM, Guo ZP, Chen Y (2016). Direct derivatization and quantitation of ultra-trace gibberellins in sub-milligram fresh plant organs. Mol Plant 9, 175-177. |
| [11] | Marquis BJ, Louks HP, Bose C, Wolfe RR, Singh SP (2017). A new derivatization reagent for HPLC-MS analy- sis of biological organic acids. Chromatographia 80, 1723-1732. |
| [12] | Pan XQ, Wang XM (2009). Profiling of plant hormones by mass spectrometry. J Chromatogr B 877, 2806-2813. |
| [13] | Pan XQ, Welti R, Wang XM (2008). Simultaneous quantifi-cation of major phytohormones and related compounds in crude plant extracts by liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69, 1773-1781. |
| [14] | Qaderi MM, Kurepin LV, Reid DM (2012). Effects of tem-perature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seed-lings. Environ Exp Bot 75, 107-113. |
| [15] | Qi BL, Liu P, Wang QY, Cai WJ, Yuan BF, Feng YQ (2014). Derivatization for liquid chromatography-mass spectro- metry. Trends Analyt Chem 59, 121-132. |
| [16] | Santa T (2011). Derivatization reagents in liquid chromato-graphy/electrospray ionization tandem mass spectrome- try. Biomed Chromatogr 25, 1-10. |
| [17] | Santa T, Al-Dirbashi OY, Fukushima T (2007). Derivatiza-tion reagents in liquid chromatography/electrospray ioni-zation tandem mass spectrometry for biomedical analysis. Drug Discov Ther 1, 108-118. |
| [18] | Santner A, Estelle M (2009). Recent advances and emerging trends in plant hormone signaling. Nature 459, 1071-1078. |
| [19] | Tank JG, Pandya RV, Thaker VS (2014). Phytohormones in regulation of the cell division and endoreduplication process in the plant cell cycle. RSC Adv 4, 12605-12613. |
| [20] | Zhang TY, Sha L, Zhu QF, Wang Q, Hussain D, Feng YQ (2019). Derivatization for liquid chromatography-electrospray ionization-mass spectrometry analysis of small-mo- lecular weight compounds. Trends Analyt Chem 119, 115608. |