TECHNIQUES AND METHODS
Detection of Reactive Oxygen Species Using H2DCFDA Probe in Plant
- College of Chemistry and Life Sciences, Zhejiang Normal University, Jinhua 321004, China
Received date: 2022-03-10
Accepted date: 2022-05-11
Online published: 2022-05-11
Abstract
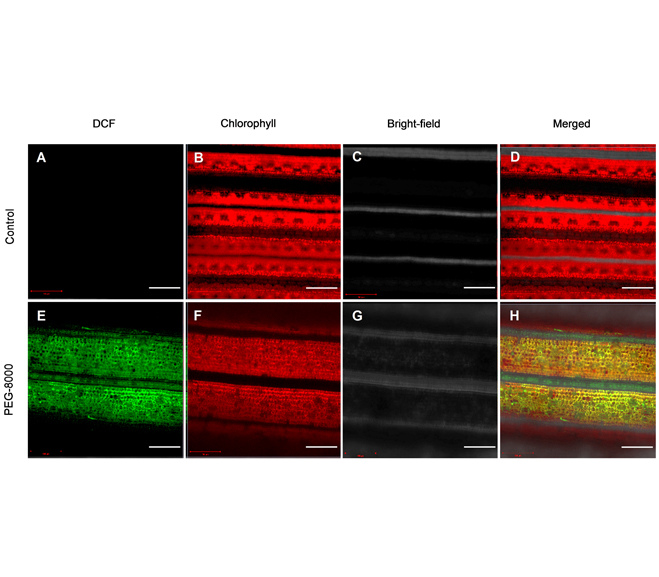
Reactive oxygen species (ROS) are a ‘double-edged sword’ in plants. On the one hand, ROS, as a signal molecule, plays pivotal roles in many aspects of life activities; on the other hand, excessive accumulation of ROS can cause oxidative damage to biological macromolecules. Accurate detection of ROS is essential to assess its intracellular redox status. Due to the characteristics of short half-life and strong reactivity of ROS components, their qualitative and quantitative analysis are difficult. It is critical to select the appropriate detection method and improve the spatiotemporal accuracy of detection for research in plant sciences and in other fields. At present, fluorescent probe analysis has attracted the attention of researchers because of its advantages of high sensitivity, good selectivity, low detection limit and strong intuition. This article introduces the detailed operation protocol and attentions for ROS detection using 2′,7′-dichlorodi-hydrofluorescein diacetate (H2DCFDA) fluorescent probe based on flow cytometry and confocal microscope. These methods can be used to detect ROS levels and distribution in model plant tissues, including Oryza sativa, Arabidopsis thaliana, Zea mays and Glycine max.
Cite this article
Haitao Hu , Tingting Qian , Ling Yang . Detection of Reactive Oxygen Species Using H2DCFDA Probe in Plant[J]. Chinese Bulletin of Botany, 2022 , 57(3) : 320 -326 . DOI: 10.11983/CBB22043
References
| [1] | Akter S, Khan MS, Smith EN, Flashman E (2021). Measuring ROS and redox markers in plant cells. RSC Chem Biol 2, 1384-1401. |
| [2] | Anjum NA, Amreen N, Tantray AY, Khan NA, Ahmad A (2020). Reactive oxygen species detection-approaches in plants: insights into genetically encoded FRET-based sen- sors. J Biotechnol 308, 108-117. |
| [3] | Apel K, Hirt H (2004). REACTIVE OXYGEN SPECIES: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55, 373-399. |
| [4] | Castro B, Citterico M, Kimura S, Stevens DM, Wrzaczek M, Coaker G (2021). Stress-induced reactive oxygen species compartmentalization, perception and signaling. Nat Plants 7, 403-412. |
| [5] | Chan ZL, Yokawa K, Kim WY, Song CP (2016). Editorial: ROS regulation during plant abiotic stress responses. Front Plant Sci 7, 1536. |
| [6] | Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J 90, 856-867. |
| [7] | Considine MJ, Foyer CH (2021a). Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol 186, 79-92. |
| [8] | Considine MJ, Foyer CH (2021b). Stress effects on the reactive oxygen species-dependent regulation of plant growth and development. J Exp Bot 72, 5795-5806. |
| [9] | Duanghathaipornsuk S, Farrell EJ, Alba-Rubio AC, Zelenay P, Kim DS (2021). Detection technologies for reactive oxygen species: fluorescence and electrochemical methods and their applications. Biosensors 11, 30. |
| [10] | Eruslanov E, Kusmartsev S (2010). Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594, 57-72. |
| [11] | Fichman Y, Miller G, Mittler R (2019). Whole-plant live imaging of reactive oxygen species. Mol Plant 12, 1203- 1210. |
| [12] | Gomes A, Fernandes E, Lima JLFC (2005). Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65, 45-80. |
| [13] | Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9, 681. |
| [14] | Hu HT, Ren DY, Hu J, Jiang HZ, Chen P, Zeng DL, Qian Q, Guo LB (2021). WHITE AND LESION-MIMIC LEAF1, encoding a lumazine synthase, affects reactive oxygen species balance and chloroplast development in rice. Plant J 108, 1690-1703. |
| [15] | Kristiansen KA, Jensen PE, Møller IM, Schulz A (2009). Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM- H2DCFDA and confocal laser microscopy. Physiol Plant 136, 369-383. |
| [16] | Li HX, Liu Y, Qin HH, Lin XL, Tang D, Wu ZJ, Luo W, Shen Y, Dong FQ, Wang YL, Feng TT, Wang LL, Li LY, Chen DD, Zhang Y, Murray JD, Chao DY, Chong K, Cheng ZK, Meng Z (2020). A rice chloroplast-localized ABC transporter ARG1 modulates cobalt and nickel homeostasis and contributes to photosynthetic capacity. New Phytol 228, 163-178. |
| [17] | Liu XY, Zhang ZG (2022). A double-edged sword: reactive oxygen species (ROS) during the rice blast fungus and host interaction. FEBS J doi:10.1111/febs.16171 |
| [18] | Maulucci G, Bačić G, Bridal L, Schmidt HH, Tavitian B, Viel T, Utsumi H, Yalçın AS, De Spirito M (2016). Imaging reactive oxygen species-induced modifications in living systems. Antioxid Redox Signal 24, 939-958. |
| [19] | Mhamdi A, van Breusegem F (2018). Reactive oxygen species in plant development. Development 145, dev164376. |
| [20] | Oparka M, Walczak J, Malinska D, van Oppen LMPE, Szczepanowska J, Koopman WJH, Wieckowski MR (2016). Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 109, 3-11. |
| [21] | Ortega-Villasante C, Burén S, Barón-Sola Á, Martínez F, Hernández LE (2016). In vivo ROS and redox potential fluorescent detection in plants: present approaches and future perspectives. Methods 109, 92-104. |
| [22] | Ortega-Villasante C, Burén S, Blázquez-Castro A, Barón- Sola Á, Hernández LE (2018). Fluorescent in vivo imaging of reactive oxygen species and redox potential in plants. Free Radic Biol Med 122, 202-220. |
| [23] | Qi JS, Song CP, Wang BS, Zhou JM, Kangasjärvi J, Zhu JK, Gong ZZ (2018). Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60, 805- 826. |
| [24] | Rajneesh, Pathak J, Chatterjee A, Singh SP, Sinha RP (2017). Detection of reactive oxygen species (ROS) in cyanobacteria using the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Bio Protoc 7, e2545. |
| [25] | Robles V, Riesco MF, Martínez-Vázquez JM, Valcarce DG (2021). Flow cytometry and confocal microscopy for ROS evaluation in fish and human spermatozoa. Methods Mol Biol 2202, 93-102. |
| [26] | Tripathy BC, Oelmüller R (2012). Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7, 1621-1633. |
| [27] | Waszczak C, Carmody M, Kangasjärvi J (2018). Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69, 209-236. |
| [28] | Xia SS, Liu H, Cui YJ, Yu HP, Rao YC, Yan YP, Zeng DL, Hu J, Zhang GH, Gao ZY, Zhu L, Shen L, Zhang Q, Li Q, Dong GJ, Guo LB, Qian Q, Ren DY (2022). UDP-N-acetylglucosamine pyrophosphorylase enhances rice survival at high temperature. New Phytol 233, 344- 359. |
| [29] | Xiong HY, Yu JP, Miao JL, Li JJ, Zhang HL, Wang X, Liu PL, Zhao Y, Jiang CH, Yin ZG, Li Y, Guo Y, Fu BY, Wang WS, Li ZK, Ali J, Li ZC (2018). Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol 178, 451-467. |
Options