

Establishment and Application of RPA-CRISPR/Cas12a Detection System for Potato Virus Y
Received date: 2021-12-23
Accepted date: 2022-03-18
Online published: 2022-03-18
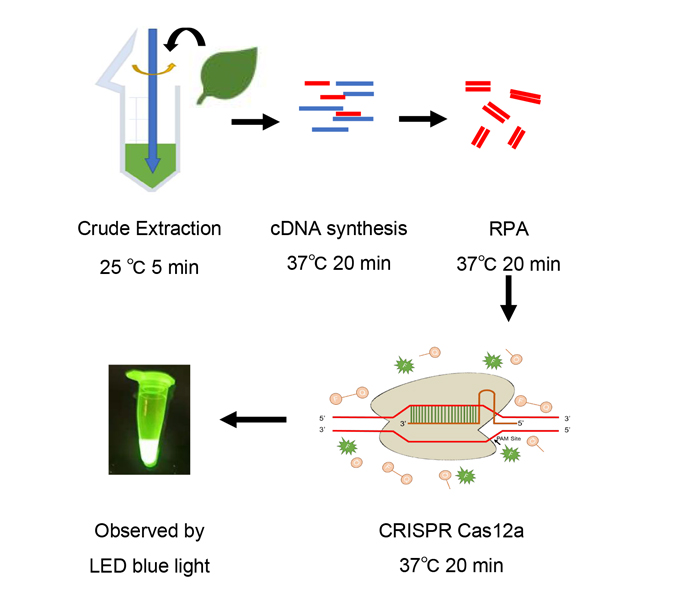
Plant virus disease is an important factor restricting the safe production of crops. Virus detection can identify viruses and determine the types of viruses, which is the key to disease monitoring, early warning and prevention in crops production. In this study, a detection system based on Recombinase Polymerase Amplification-Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated 12a (RPA-CRISPR/Cas12a) was established for potato virus Y (PVY). The results showed that: (1) Cas12a and other components in the CRISPR/Cas12a detection system were necessary for the detection; (2) The target location of crRNA had a significant effect on Cas12a nuclease activity, and the reaction efficiency was the highest when the target of crRNA contained part of PAM site sequence; (3) The minimum detection limit of RPA-CRISPR/Cas12a was 3×102 copies∙μL-1, which was higher than that of PCR and qPCR methods; (4) The combination of RPA-CRISPR/Cas12a system with crude extraction of nucleic acid and reverse transcription could detect PVY in a non-laboratory setting and the whole process took about 60 minutes. The RPA-CRISPR/Cas12a detection system of PVY established in this study provides an effective method for real-time and rapid visual detection of plant viruses under non-laboratory conditions.
Yulong He , Jiage Wang , Shanshan Zhao , Jin Gao , Yingying Chang , Xiting Zhao , Bihua Nie , Qingxiang Yang , Jiangli Zhang , Mingjun Li . Establishment and Application of RPA-CRISPR/Cas12a Detection System for Potato Virus Y[J]. Chinese Bulletin of Botany, 2022 , 57(3) : 308 -319 . DOI: 10.11983/CBB21225
| [1] | 董芳娟, 魏增云, 薄一览 (2021). 分子生物学技术在植物病毒检测中的应用. 农业与技术 41(11), 23-25. |
| [2] | 方琦, 董家红, 郑宽瑜, 张仲凯 (2014). 番茄环纹斑点病毒与马铃薯Y病毒复合侵染烟草的细胞病理特征. 植物学报 49, 704-709. |
| [3] | 李明军, 张峰, 陈明霞, 于相丽 (2003). 怀山药病毒病的研究. 中草药 11, 100-102. |
| [4] | 罗雪琮, 安梦楠, 吴元华, 夏子豪 (2022). 重组酶聚合酶扩增技术在植物病毒检测中的应用. 生物技术通报 38, 269-280. |
| [5] | 王亚楠, 陈昌国 (2021). 重组酶聚合酶扩增技术研究进展. 解放军医学杂志 46, 504-511. |
| [6] | 王玉英, 高新一 (2006). 植物组织培养技术手册. 北京: 金盾出版社. pp. 121-148. |
| [7] | 王紫怡, 黄迪, 徐颖华, 黄磊, 连佳长, 徐志南 (2021). CRISPR核酸检测技术的研究进展. 食品安全质量检测学报 12, 6711-6719. |
| [8] | 杨小龙, 陈细红, 蔡伟, 高芳銮, 沈建国, 吴祖建 (2019). 福建省福清地区马铃薯病毒病病原的分子检测. 植物保护 45(3), 201-205. |
| [9] | 游旸, 杜宗敏 (2021). 基于CRISPR-Cas系统的核酸检测方法研究进展. 军事医学 45, 955-960. |
| [10] | 余武秀, 申继忠 (2021). 植物病毒病和抗病毒剂. 世界农药 43(5), 17-24. |
| [11] | 张静雅, 何衍彪 (2019). 植物病毒病检测及防治技术研究进展. 安徽农学通报 25(12), 79-81, 83. |
| [12] | Ding X, Yin K, Li ZY, Lalla RV, Ballesteros E, Sfeir MM, Liu CC (2020). Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat Commun 11, 4711. |
| [13] | Jiao J, Kong KK, Han JM, Song SW, Bai TH, Song CH, Wang MM, Yan ZL, Zhang HT, Zhang RP, Feng JC, Zheng XB (2021). Field detection of multiple RNA viruses/viroids in apple using a CRISPR/Cas12a-based visual assay. Plant Biotechnol J 19, 394-405. |
| [14] | Kang HX, Peng Y, Hua KY, Deng YF, Bellizzi M, Gupta DR, Mahmud NU, Urashima AS, Paul SK, Peterson G, Zhou YL, Zhou XP, Islam T, Wang GL (2021). Rapid detection of wheat blast pathogen Magnaporthe oryzae triticum pathotype using genome-specific primers and Cas12a-mediated technology. Engineering 7, 1326-1335. |
| [15] | Liu H, Wang JB, Zeng HJ, Liu XF, Jiang W, Wang Y, Ouyang WB, Tang XM (2021). RPA-Cas12a-FS: a frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem 334, 127608. |
| [16] | Silva G, Oyekanmi J, Nkere CK, Bömer M, Kumar PL, Seal SE (2018). Rapid detection of potyviruses from crude plant extracts. Anal Biochem 546, 17-22. |
| [17] | Swarts DC, Jinek M (2019). Mechanistic insights into the cis- and trans-acting DNase activities of Cas12a. Mol Cell 73, 589-600. |
| [18] | Wang B, Wang R, Wang DQ, Wu J, Li JX, Wang J, Liu HH, Wang YM (2019). Cas12aVDet: a CRISPR/Cas12a-based platform for rapid and visual nucleic acid detection. Anal Chem 91, 12156-12161. |
| [19] | Wang Y, Chen RH, Nie XZ, Zhong ZY, Li CY, Li K, Huang W, Fu XY, Liu J, Nie BH (2020). Rapid and sensitive detection of potato virus Y by isothermal reverse transcription-recombinase polymerase amplification assay in potato. Mol Cell Probes 50, 101505. |
| [20] | Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F (2015). Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759-771. |
/
| 〈 |
|
〉 |