

Research Advances in Oxysterol-binding Protein-related Proteins
Received date: 2021-03-09
Accepted date: 2021-05-27
Online published: 2021-05-27
Membrane lipids not only are important components of cell membranes, but also participate in signal transduction as signal molecules. The uneven distribution of lipid molecules in membranes requires specific types of transport channels and transporters for each lipid type. Oxysterol-binding protein (OSBP)-related proteins (ORPs) are a highly conserved family of lipid transport proteins that recognize and transport phosphoinositides and sterols, which are involved in many physiological processes including signal transduction, vesicle transport, lipid metabolism and non-vesicle transport, and hence play a very important role in the growth and development of individual organism. In recent years, a series of important findings have been made on the structure and function of ORPs in mammals and yeasts, but the advances in plants are relatively slow. In this paper, we review the progress of ORPs research in mammals, yeasts and plants, analyze the structural domains of ORPs in plants and the phylogenetical relationship to their homologs in mammals and yeasts, and also provide perspectives on the directions of plant ORPs research in the future.
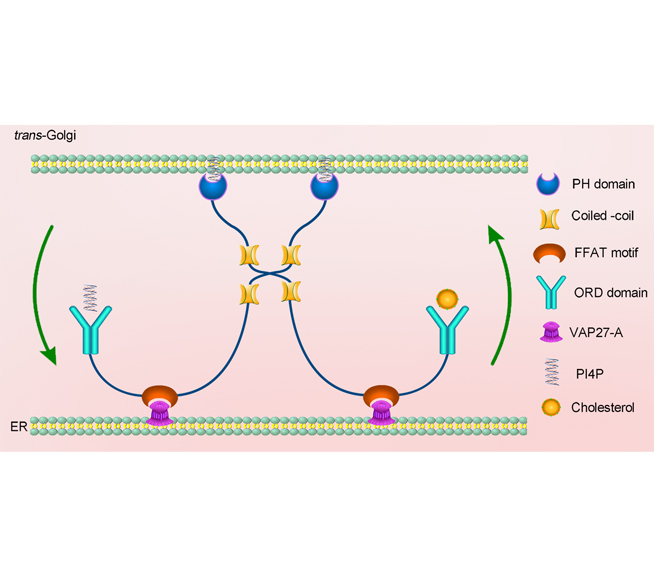
Wenjiao Zou, Lei Ge, Qian Yu . Research Advances in Oxysterol-binding Protein-related Proteins[J]. Chinese Bulletin of Botany, 2021 , 56(5) : 627 -640 . DOI: 10.11983/CBB21045
| [1] | Amarilio R, Ramachandran S, Sabanay H, Lev S (2005). Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem 280, 5934-5944. |
| [2] | Anniss AM, Apostolopoulos J, Dworkin S, Purton LE, Sparrow RL (2002). An oxysterol-binding protein family identified in the mouse. DNA Cell Biol 21, 571-580. |
| [3] | Balla T, Bondeva T, Varnai P (2000). How accurately can we image inositol lipids in living cells? Trends Pharmacol Sci 21, 238-241. |
| [4] | Balla T, Kim YJ, Alvarez-Prats A, Pemberton J (2019). Lipid dynamics at contact sites between the endoplasmic reticulum and other organelles. Annu Rev Cell Dev Biol 35, 85-109. |
| [5] | Beh CT, Cool L, Phillips J, Rine J (2001). Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics 157, 1117-1140. |
| [6] | Beh CT, Rine J (2004). A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci 117, 2983-2996. |
| [7] | Berendzen KW, Böhmer M, Wallmeroth N, Peter S, Vesić M, Zhou Y, Tiesler FKE, Schleifenbaum F, Harter K (2012). Screening for in planta protein-protein interactions combining bimolecular fluorescence complementation with flow cytometry. Plant Methods 8, 25. |
| [8] | Boutté Y, Grebe M (2009). Cellular processes relying on sterol function in plants. Curr Opin Plant Biol 12, 705-713. |
| [9] | Chen HJ, Anagnostou G, Chai A, Withers J, Morris A, Adhikaree J, Pennetta G, de Belleroche JS (2010). Characterization of the properties of a novel mutation in VAPB in familial amyotrophic lateral sclerosis. J Biol Chem 285, 40266-40281. |
| [10] | Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P (2015). PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349, 428-432. |
| [11] | Chung T (2019). How phosphoinositides shape autophagy in plant cells. Plant Sci 281, 146-158. |
| [12] | D'Angelo G, Vicinanza M, De Matteis MA (2008). Lipid- transfer proteins in biosynthetic pathways. Curr Opin Cell Biol 20, 360-370. |
| [13] | De Craene JO, Bertazzi DL, Bär S, Friant S (2017). Phosphoinositides, major actors in membrane trafficking and lipid signaling pathways. Int J Mol Sci 18, 634. |
| [14] | De Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G (2011). Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol 195, 965-978. |
| [15] | Deeks MJ, Calcutt JR, Ingle EKS, Hawkins TJ, Chapman S, Richardson AC, Mentlak DA, Dixon MR, Cartwright F, Smertenko AP, Oparka K, Hussey PJ (2012). A superfamily of actin-binding proteins at the actin-membrane nexus of higher plants. Curr Biol 22, 1595-1600. |
| [16] | Dong R, Saheki Y, Swarup S, Lucast L, Harper JW, De Camilli P (2016). Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell 166, 408-423. |
| [17] | Du XM, Kumar J, Ferguson C, Schulz TA, Ong YS, Hong WJ, Prinz WA, Parton RG, Brown AJ, Yang HY (2011). A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J Cell Biol 192, 121-135. |
| [18] | Eisenberg-Bord M, Shai N, Schuldiner M, Bohnert M (2016). A tether is a tether is a tether: tethering at membrane contact sites. Dev Cell 39, 395-409. |
| [19] | Ferrer A, Altabella T, Arró M, Boronat A (2017). Emerging roles for conjugated sterols in plants. Prog Lipid Res 67, 27-37. |
| [20] | Friedman JR, Voeltz GK (2011). The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21, 709-717. |
| [21] | Galmes R, Houcine A, van Vliet AR, Agostinis P, Jackson CL, Giordano F (2016). ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Rep 17, 800-810. |
| [22] | Gatta AT, Levine TP (2017). Piecing together the patchwork of contact sites. Trends Cell Biol 27, 214-229. |
| [23] | Geldner N, Dénervaud-Tendon V, Hyman DL, Mayer U, Stierhof YD, Chory J (2009). Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59, 169-178. |
| [24] | Geuns JMC (1983). Plant steroid hormones. Biochem Soc Trans 11, 543-548. |
| [25] | Ghai R, Du XM, Wang H, Dong JQ, Ferguson C, Brown AJ, Parton RG, Wu JW, Yang HY (2017). ORP5 and ORP8 bind phosphatidylinositol-4,5-biphosphate (PtdIns (4,5)P2) and regulate its level at the plasma membrane. Nat Commun 8, 757. |
| [26] | Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA (2004). FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6, 393-404. |
| [27] | Guimil S, Dunand C (2007). Cell growth and differentiation in Arabidopsis epidermal cells. J Exp Bot 58, 3829-3840. |
| [28] | Gulyás G, Sohn M, Kim YJ, Várnai P, Balla T (2020). ORP3 phosphorylation regulates phosphatidylinositol 4-phosphate and Ca2+ dynamics at plasma membrane-ER contact sites. J Cell Sci 133, jcs237388. |
| [29] | Harayama T, Riezman H (2018). Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol 19, 281-296. |
| [30] | Heilmann I (2016). Phosphoinositide signaling in plant development. Development 143, 2044-2055. |
| [31] | Helle SCJ, Kanfer G, Kolar K, Lang A, Michel AH, Kornmann B (2013). Organization and function of membrane contact sites. Biochim Biophys Acta Mol Cell Res 1833, 2526-2541. |
| [32] | Hong YY, Zhao J, Guo L, Kim SC, Deng XJ, Wang GL, Zhang GY, Li MY, Wang XM (2016). Plant phospholipases D and C and their diverse functions in stress responses. Prog Lipid Res 62, 55-74. |
| [33] | Hynynen R, Suchanek M, Spandl J, Bäck N, Thiele C, Olkkonen VM (2009). OSBP-related protein 2 is a sterol receptor on lipid droplets that regulates the metabolism of neutral lipids. J Lipid Res 50, 1305-1315. |
| [34] | Ishikawa S, Nagai Y, Masuda T, Koga Y, Nakamura T, Imamura Y, Takamori H, Hirota M, Funakosi A, Fukushima M, Baba H (2010). The role of oxysterol binding protein-related protein 5 in pancreatic cancer. Cancer Sci 101, 898-905. |
| [35] | Jansen M, Ohsaki Y, Rega LR, Bittman R, Olkkonen VM, Ikonen E (2011). Role of ORPs in sterol transport from plasma membrane to ER and lipid droplets in mammalian cells. Traffic 12, 218-231. |
| [36] | Jaworski CJ, Moreira E, Li AG, Lee R, Rodriguez IR (2001). A family of 12 human genes containing oxysterol-binding domains. Genomics 78, 185-196. |
| [37] | Jia Q, Kong DF, Li QH, Sun S, Song JL, Zhu YB, Liang KL, Ke QM, Lin WX, Huang JW (2019). The function of inositol phosphatases in plant tolerance to abiotic stress. Int J Mol Sci 20, 3999. |
| [38] | Johansson M, Lehto M, Tanhuanpää K, Cover TL, Olkkonen VM (2005). The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol Biol Cell 16, 5480-5492. |
| [39] | Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J (2007). Activation of endosomal dynein motors by stepwise assembly of Rab7- RILP-p150Glued, ORP1L, and the receptorβlll spectrin. J Cell Biol 176, 459-471. |
| [40] | Kabashi E, El Oussini H, Bercier V, Gros-Louis F, Valdmanis PN, McDearmid J, Mejier IA, Dion PA, Dupre N, Hollinger D, Sinniger J, Dirrig-Grosch S, Camu W, Meininger V, Loeffler JP, René F, Drapeau P, Rouleau GA, Dupuis L (2013). Investigating the contribution of VAPB/ALS8 loss of function in amyotrophic lateral sclerosis. Hum Mol Genet 22, 2350-2360. |
| [41] | Kagiwada S, Hashimoto M (2007). The yeast VAP homolog Scs2p has a phosphoinositide-binding ability that is correlated with its activity. Biochem Biophys Res Commun 364, 870-876. |
| [42] | Kagiwada S, Zen R (2003). Role of the yeast VAP homolog, Scs2p, in INO1 expression and phospholipid metabolism. J Biochem 133, 515-522. |
| [43] | Kandutsch AA, Thompson EB (1980). Cytosolic proteins that bind oxygenated sterols. Cellular distribution, specificity, and some properties. J Biol Chem 255, 10813-10821. |
| [44] | Kawano M, Kumagai K, Nishijima M, Hanada K (2006). Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem 281, 30279-30288. |
| [45] | Ketelaar T, Allwood EG, Hussey PJ (2007). Actin organization and root hair development are disrupted by ethanol-induced overexpression of Arabidopsis actin interacting protein 1 (AIP1). New Phytol 174, 57-62. |
| [46] | Kobuna H, Inoue T, Shibata M, Gengyo-Ando K, Yamamoto A, Mitani S, Arai H (2010). Multivesicular body formation requires OSBP-related proteins and cholesterol. PLoS Genet 6, e1001055. |
| [47] | Kun-Rodrigues C, Ganos C, Guerreiro R, Schneider SA, Schulte C, Lesage S, Darwent L, Holmans P, Singleton A, International Parkinson's Disease Genomics Consortium (IPDGC), Bhatia K, Bras J (2015). A systematic screening to identify de novo mutations causing sporadic early-onset Parkinson’s disease. Hum Mol Genet 24, 6711-6720. |
| [48] | Lagace TA, Byers DM, Cook HW, Ridgway ND (1997). Altered regulation of cholesterol and cholesteryl ester synthesis in Chinese-hamster ovary cells overexpressing the oxysterol-binding protein is dependent on the pleckstrin homology domain. Biochem J 326(Pt1), 205-213. |
| [49] | Lehto M, Laitinen S, Chinetti G, Johansson M, Ehnholm C, Staels B, Ikonen E, Olkkonen VM (2001). The OSBP-related protein family in humans. J Lipid Res 42, 1203-1213. |
| [50] | Lemmon MA (2003). Phosphoinositide recognition domains. Traffic 4, 201-213. |
| [51] | Lemmon MA (2007). Pleckstrin homology (PH) domains and phosphoinositides. Biochem Soc Symp 74, 81-93. |
| [52] | Lessmann E, Ngo M, Leitges M, Minguet S, Ridgway ND, Huber M (2007). Oxysterol-binding protein-related protein (ORP) 9 is a PDK-2 substrate and regulates Akt phosphorylation. Cell Signal 19, 384-392. |
| [53] | Lev S, Halevy DB, Peretti D, Dahan N (2008). The VAP protein family: from cellular functions to motor neuron disease. Trends Cell Biol 18, 282-290. |
| [54] | Levine T (2004). Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol 14, 483-490. |
| [55] | Levine TP, Munro S (2001). Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. Mol Biol Cell 12, 1633-1644. |
| [56] | Levine TP, Munro S (2002). Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase- dependent and-independent components. Curr Biol 12, 695-704. |
| [57] | Li DY, Inoue H, Takahashi M, Kojima T, Shiraiwa M, Takahara H (2008). Molecular characterization of a novel salt-inducible gene for an OSBP (oxysterol-binding protein)-homologue from soybean. Gene 407, 12-20. |
| [58] | Li ZC, He YH (2020). Roles of brassinosteroids in plant reproduction. Int J Mol Sci 21, 872. |
| [59] | Liu XW, Ridgway ND (2014). Characterization of the sterol and phosphatidylinositol 4-phosphate binding properties of Golgi-associated OSBP-related protein 9 (ORP9). PLoS One 9, e108368. |
| [60] | Liu Y, Su Y, Wang XM (2013). Phosphatidic acid-mediated signaling. Adv Exp Med Biol 991, 159-176. |
| [61] | Loewen CJR, Roy A, Levine TP (2003). A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J 22, 2025-2035. |
| [62] | Lundbæk JA, Collingwood SA, Ingólfsson HI, Kapoor R, Andersen OS (2010). Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface 7, 373-395. |
| [63] | Luo J, Yang HY, Song BL (2020). Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol 21, 225-245. |
| [64] | Ma XH, Shor O, Diminshtein S, Yu L, Im YJ, Perera I, Lomax A, Boss WF, Moran N (2009). Phosphatidylinositol (4,5) bisphosphate inhibits K+-efflux channel activity in NT1 tobacco cultured cells. Plant Physiol 149, 1127-1140. |
| [65] | Ma ZG, Liu ZH, Huang X (2010). OSBP- and FAN-mediated sterol requirement for spermatogenesis in Drosophila. Development 137, 3775-3784. |
| [66] | Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, Gavin AC (2013). Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature 501, 257-261. |
| [67] | Malinsky J, Opekarova M, Grossmann G, Tanner W (2013). Membrane microdomains, rafts, and detergent- resistant membranes in plants and fungi. Annu Rev Plant Biol 64, 501-529. |
| [68] | Manik MK, Yang H, Tong J, Im YJ (2017). Structure of yeast OSBP-related protein OSH1 reveals key determinants for lipid transport and protein targeting at the nucleus-vacuole junction. Structure 25, 617-629. |
| [69] | Mao DX, Lin G, Tepe B, Zuo ZY, Tan KL, Senturk M, Zhang S, Arenkiel BR, Sardiello M, Bellen HJ (2019). VAMP associated proteins are required for autophagic and lysosomal degradation by promoting a PtdIns4P-medIated endosomal pathway. Autophagy 15, 1214-1233. |
| [70] | Martin TF (2015). PI(4,5)P2-binding effector proteins for vesicle exocytosis. Biochim Biophys Acta Mol Cell Biol Lipids 1851, 785-793. |
| [71] | Mesmin B, Bigay J, Polidori J, Jamecna D, Lacas-Ger-vais S, Antonny B (2017). Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J 36, 3156-3174. |
| [72] | Mesmin B, Bigay J, von Filseck JM, Lacas-Gervais S, Drin G, Antonny B (2013). A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155, 830-843. |
| [73] | Mochizuki S, Miki H, Zhou RY, Kido Y, Nishimura W, Kikuchi M, Noda Y (2018). Oxysterol-binding protein- related protein (ORP) 6 localizes to the ER and ER- plasma membrane contact sites and is involved in the turnover of PI4P in cerebellar granule neurons. Exp Cell Res 370, 601-612. |
| [74] | Moreau P, Hartmann MA, Perret AM, Sturbois-Balcerzak B, Cassagne C (1998). Transport of sterols to the plasma membrane of leek seedlings. Plant Physiol 117, 931-937. |
| [75] | Murphy SE, Levine TP (2016). VAP, a versatile access point for the endoplasmic reticulum: review and analysis of FFAT-like motifs in the VAPome. Biochim Biophys Acta Mol Cell Biol Lipids 1861, 952-961. |
| [76] | Ngo M, Ridgway ND (2009). Oxysterol binding protein- related protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol Biol Cell 20, 1388-1399. |
| [77] | Nishimura AL, Mitne-Neto M, Silva HCA, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JRM, Gillingwater T, Webb J, Skehel P, Zatz M (2004). A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75, 822-831. |
| [78] | Nishimura Y, Hayashi M, Inada H, Tanaka T (1999). Molecular cloning and characterization of mammalian homologues of vesicle-associated membrane protein-assocIated (VAMP-associated) proteins. Biochem Biophys Res Commun 254, 21-26. |
| [79] | Petersen NH, Joensen J, McKinney LV, Brodersen P, Petersen M, Hofius D, Mundy J (2009). Identification of proteins interacting with Arabidopsis ACD11. J Plant Physiol 166, 661-666. |
| [80] | Pietrangelo A, Ridgway ND (2018). Bridging the molecular and biological functions of the oxysterol-binding protein family. Cell Mol Life Sci 75, 3079-3098. |
| [81] | Raychaudhuri S, Im YJ, Hurley JH, Prinz WA (2006). Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol 173, 107-119. |
| [82] | Raychaudhuri S, Prinz WA (2010). The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol 26, 157-177. |
| [83] | Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J (2009). Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150Glued and late endosome positioning. J Cell Biol 185, 1209-1225. |
| [84] | Roy A, Levine TP (2004). Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J Biol Chem 279, 44683-44689. |
| [85] | Rusten TE, Stenmark H (2006). Analyzing phosphoinositides and their interacting proteins. Nat Methods 3, 251-258. |
| [86] | Saheki Y, De Camilli P (2017). Endoplasmic reticulum- plasma membrane contact sites. Annu Rev Biochem 86, 659-684. |
| [87] | Saravanan RS, Slabaugh E, Singh VR, Lapidus LJ, Haas T, Brandizzi F (2009). The targeting of the oxysterol- binding protein ORP3a to the endoplasmic reticulum relies on the plant VAP33 homolog PVA12. Plant J 58, 817-830. |
| [88] | Schaaf G, Ortlund EA, Tyeryar KR, Mousley CJ, Ile KE, Garrett TA, Ren JH, Woolls MJ, Raetz CRH, Redinbo MR, Bankaitis VA (2008). Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol Cell 29, 191-206. |
| [89] | Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA (2009). Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol 187, 889-903. |
| [90] | Skehel PA, Martin KC, Kandel ER, Bartsch D (1995). A VAMP-binding protein from Aplysia required for neurotransmitter release. Science 269, 1580-1583. |
| [91] | Skirpan AL, Dowd PE, Sijacic P, Jaworski CJ, Gilroy S, Kao TH (2006). Identification and characterization of PiORP1, a Petunia oxysterol-binding-protein related protein involved in receptor-kinase mediated signaling in pollen, and analysis of the ORP gene family in Arabidopsis. Plant Mol Biol 61, 553-565. |
| [92] | Soussan L, Burakov D, Daniels MP, Toister-Achituv M, Porat A, Yarden Y, Elazar Z (1999). ERG30, a VAP-33- related protein, functions in protein transport mediated by COPI vesicles. J Cell Biol 146, 301-312. |
| [93] | Stefano G, Renna L, Wormsbaecher C, Gamble J, Zienkiewicz K, Brandizzi F (2018). Plant endocytosis requires the ER membrane-anchored proteins VAP27-1 and VAP27-3. Cell Rep 23, 2299-2307. |
| [94] | Takáč T, Novák D, Šamaj J (2019). Recent advances in the cellular and developmental biology of phospholipases in plants. Front Plant Sci 10, 362. |
| [95] | Takahashi D, Kawamura Y, Uemura M (2013). Changes of detergent-resistant plasma membrane proteins in oat and rye during cold acclimation: association with differential freezing tolerance. J Proteome Res 12, 4998-5011. |
| [96] | Tapken W, Murphy AS (2015). Membrane nanodomains in plants: capturing form, function, and movement. J Exp Bot 66, 1573-1586. |
| [97] | Tarkowská D (2019). Plants are capable of synthesizing animal steroid hormones. Molecules 24, 2585. |
| [98] | Taylor FR, Kandutsch AA (1985). [2] Use of oxygenated sterols to probe the regulation of 3-hydroxy-3-methylglutaryl-CoA reductase and sterologenesis. Methods Enzymol 110, 9-19. |
| [99] | Taylor FR, Saucier SE, Shown EP, Parish EJ, Kandutsch AA (1984). Correlation between oxysterol binding to a cytosolic binding protein and potency in the repression of hydroxymethylglutaryl coenzyme a reductase. J Biol Chem 259, 12382-12387. |
| [100] | Tong JS, Yang H, Yang HY, Eom SH, Im YJ (2013). Structure of Osh3 reveals a conserved mode of phosphoinositide binding in oxysterol-binding proteins. Structure 21, 1203-1213. |
| [101] | van der Kant R, Fish A, Janssen L, Janssen H, Krom S, Ho N, Brummelkamp T, Carette J, Rocha N, Neefjes J (2013). Late endosomal transport and tethering are coupled processes controlled by RILP and the cholesterol sensor ORP1L. J Cell Sci 126, 3462-3474. |
| [102] | van Meer G, Voelker DR, Feigenson GW (2008). Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9, 112-124. |
| [103] | Vihervaara T, Uronen RL, Wohlfahrt G, Björkhem I, Ikonen E, Olkkonen VM (2011). Sterol binding by OSBP- related protein 1L regulates late endosome motility and function. Cell Mol Life Sci 68, 537-551. |
| [104] | von Filseck JM, Čopič A, Delfosse V, Vanni S, Jackson CL, Bourguet W, Drin G (2015a). Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 349, 432-436. |
| [105] | von Filseck JM, Vanni S, Mesmin B, Antonny B, Drin G (2015b). A phosphatidylinositol-4-phosphate powered exchange mechanism to create a lipid gradient between membranes. Nat Commun 6, 6671. |
| [106] | Wang H, Ma QL, Qi YF, Dong JQ, Du XM, Rae J, Wang J, Wu WF, Brown AJ, Parton RG, Wu JW, Yang HY (2019a). ORP2 delivers cholesterol to the plasma membrane in exchange for phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). Mol Cell 73, 458-473. |
| [107] | Wang PH, Duan W, Munn AL, Yang HY (2005a). Molecular characterization of Osh6p, an oxysterol binding protein homolog in the yeast Saccharomyces cerevisiae. FEBS J 272, 4703-4715. |
| [108] | Wang PW, Hawkins TJ, Richardson C, Cummins I, Deeks MJ, Sparkes I, Hawes C, Hussey PJ (2014). The plant cytoskeleton, NET3C, and VAP27 mediate the link between the plasma membrane and endoplasmic reticulum. Curr Biol 24, 1397-1405. |
| [109] | Wang PW, Pleskot R, Zang JZ, Winkler J, Wang J, Yperman K, Zhang T, Wang K, Gong JL, Guan YJ, Richardson C, Duckney P, Vandorpe M, Mylle E, Fiserova J, Van Damme D, Hussey PJ (2019b). Plant AtEH/Pan1 proteins drive autophagosome formation at ER-PM contact sites with actin and endocytic machinery. Nat Commun 10, 5132. |
| [110] | Wang PW, Richardson C, Hawkins TJ, Sparkes I, Hawes C, Hussey PJ (2016a). Plant VAP27 proteins: domain characterization, intracellular localization and role in plant development. New Phytol 210, 1311-1326. |
| [111] | Wang PY, Weng J, Anderson RGW (2005b). OSBP is a cholesterol-regulated scaffolding protein in control of ERK1/2 activation. Science 307, 1472-1476. |
| [112] | Wang WM, Liu PQ, Xu YJ, Xiao SY (2016b). Protein trafficking during plant innate immunity. J Integr Plant Biol 58, 284-298. |
| [113] | Weber-Boyvat M, Kentala H, Lilja J, Vihervaara T, Hanninen R, Zhou Y, Peränen J, Nyman TA, Ivaska J, Olkkonen VM (2015a). OSBP-related protein 3 (ORP3) coupling with VAMP-associated protein A regulates R-Ras activity. Exp Cell Res 331, 278-291. |
| [114] | Weber-Boyvat M, Kentala H, Peränen J, Olkkonen VM (2015b). Ligand-dependent localization and function of ORP-VAP complexes at membrane contact sites. Cell Mol Life Sci 72, 1967-1987. |
| [115] | Wijdeven RH, Janssen H, Nahidiazar L, Janssen L, Jalink K, Berlin I, Neefjes J (2016). Cholesterol and ORP1L- mediated ER contact sites control autophagosome transport and fusion with the endocytic pathway. Nat Commun 7, 11808. |
| [116] | Wong LH, Gatta AT, Levine TP (2019). Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat Rev Mol Cell Biol 20, 85-101. |
| [117] | Wyles JP, McMaster CR, Ridgway ND (2002). Vesicle- associated membrane protein-associated protein-A (VAP- A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem 277, 29908-29918. |
| [118] | Wyles JP, Perry RJ, Ridgway ND (2007). Characterization of the sterol-binding domain of oxysterol-binding protein (OSBP)-related protein 4 reveals a novel role in vimentin organization. Exp Cell Res 313, 1426-1437. |
| [119] | Wyles JP, Ridgway ND (2004). VAMP-associated protein-A regulates partitioning of oxysterol-binding protein-related protein-9 between the endoplasmic reticulum and Golgi apparatus. Exp Cell Res 297, 533-547. |
| [120] | Xing JJ, Zhang L, Duan ZK, Lin JX (2021). Coordination of phospholipid-based signaling and membrane trafficking in plant immunity. Trends Plant Sci 26, 407-420. |
| [121] | Xue HW, Chen X, Mei Y (2009). Function and regulation of phospholipid signaling in plants. Biochem J 421, 145-156. |
| [122] | Yan DG, Lehto M, Rasilainen L, Metso J, Ehnholm C, Ylä-Herttuala S, Jauhiainen M, Olkkonen VM (2007). Oxysterol binding protein induces upregulation of SREBP- 1c and enhances hepatic lipogenesis. Arterioscler Thromb Vasc Biol 27, 1108-1114. |
| [123] | Yan DG, Ma?yra?npa?a? MI, Wong J, Perttila? J, Lehto M, Jauhiainen M, Kovanen PT, Ehnholm C, Brown AJ, Olkkonen VM (2008). OSBP-related protein 8 (ORP8) suppresses ABCA1 expression and cholesterol efflux from macrophages. J Biol Chem 283, 332-340. |
| [124] | Ye H, Ji CY, Guo RF, Jiang LW (2020). Membrane contact sites and organelles interaction in plant autophagy. Front Plant Sci 11, 477. |
| [125] | Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA (2004). Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell 13, 677-688. |
| [126] | Zauber H, Burgos A, Garapati P, Schulze WX (2014). Plasma membrane lipid-protein interactions affect signaling processes in sterol-biosynthesis mutants in Arabidopsis thaliana. Front Plant Sci 5, 78. |
| [127] | Zhou Y, Li SQ, Mäyränpää MI, Zhong WB, Bäck N, Yan DG, Olkkonen VM (2010). OSBP-related protein 11 (ORP11) dimerizes with ORP9 and localizes at the Golgi- late endosome interface. Exp Cell Res 316, 3304-3316. |
| [128] | Zhu DM, Zhang MD, Gao CJ, Shen JB (2020). Protein trafficking in plant cells: tools and markers. Sci China Life Sci 63, 343-363. |
| [129] | Zhuang XH, Chung KP, Cui Y, Lin WL, Gao CJ, Kang BH, Jiang LW (2017). ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc Natl Acad Sci USA 114, E426-E435. |
/
| 〈 |
|
〉 |