

Influence Mechanisms of Nitric Oxide on Nodulation and Nitrogen Fixation in Legumes
Received date: 2020-03-03
Accepted date: 2020-06-05
Online published: 2020-06-05
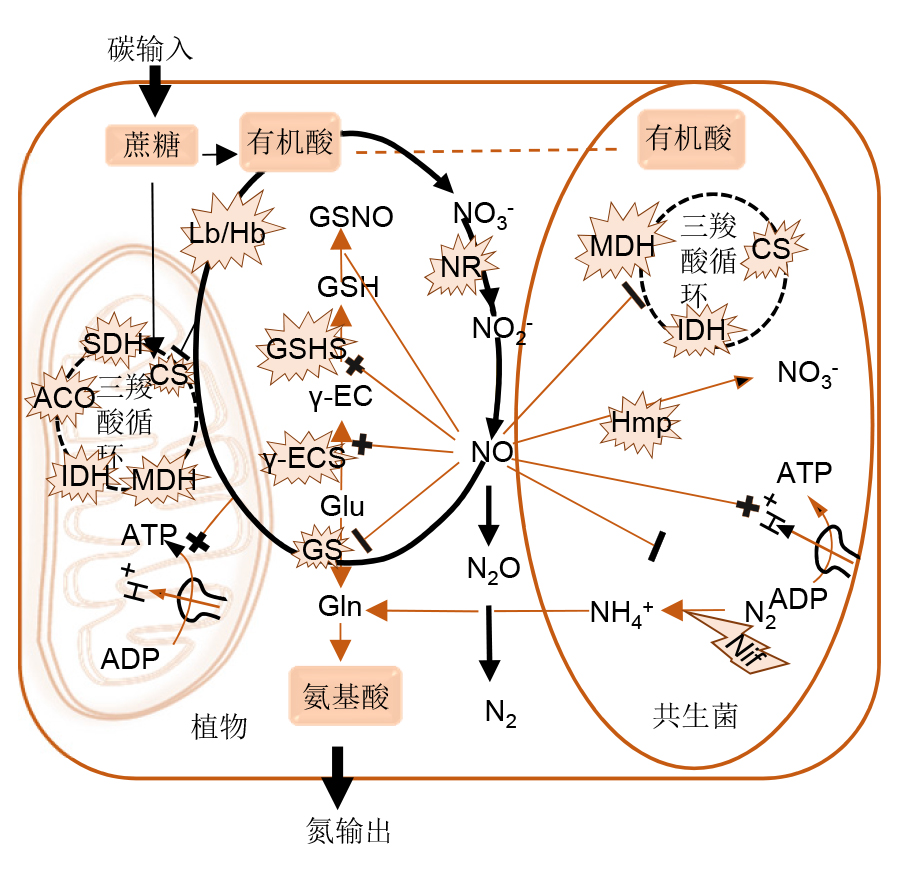
Legume-rhizobium symbiosis is genetically co-regulated by the genes of both partners. The symbiosis process involves the formation of special nodule structure where the inert nitrogen (N2) from the atmosphere is converted into ammonia nitrogen that can be directly used by plants. Nodulation and nitrogen fixation are affected by many factors. As a free radical reactive gas signaling molecule, nitric oxide (NO) participates in the regulation of many plant growth and development processes, such as respiration, photomorphogenesis, seed germination, tissue and organ development, aging, and response to various biotic and abiotic stresses. In the legumes, it has been found that NO not only affects the establishment of the symbiotic relationship between the host and the bacteria, but also is involved in regulating the fixation of nitrogen by the rhizobia and increases the efficiency of nitrogen nutrition utilization. Here we review the mechanism of NO regulating nodule formation and symbiotic nitrogen fixation in legume-rhizobium symbiosis system, including the production and degradation of NO in legumes and rhizobia and its effect on nodulation, symbiotic nitrogen fixation and their response to environmental stress. We discuss the prospects and challenges of studying NO signaling molecule in symbiotic nitrogen fixation system of legume-rhizobium.
Key words: nitric oxide (NO); nodule; symbiotic nitrogen fixation; hemoglobins (Hbs)
Weiqin Zhang , Hang Zou , Nina Zhang , Xueyuan Lin , Juan Chen . Influence Mechanisms of Nitric Oxide on Nodulation and Nitrogen Fixation in Legumes[J]. Chinese Bulletin of Botany, 2020 , 55(5) : 623 -633 . DOI: 10.11983/CBB20034
| [1] | 何恒斌, 贾桂霞 ( 2013). 豆科植物早期共生信号转导的研究进展. 植物学报 48, 665-675. |
| [2] | 李欣欣, 许锐能, 廖红 ( 2016). 大豆共生固氮在农业减肥增效中的贡献及应用潜力. 大豆科学 35, 531-535. |
| [3] | 尚玉婷, 张妮娜, 上官周平, 陈娟 ( 2018). 硫化氢在植物中的生理功能及作用机制. 植物学报 53, 565-574. |
| [4] | 张绪成, 上官周平, 高世铭 ( 2005). NO对植物生长发育的调控机制. 西北植物学报 25, 812-818. |
| [5] | Arjona D, Wikstr?m M, ?delroth P ( 2015). Nitric oxide is a potent inhibitor of the cbb3-type heme-copper oxidases. FEBS Lett 589, 1214-1218. |
| [6] | Bartnikas TB, Wang YS, Bobo T, Veselo A, Scholes CP, Shapleigh JP ( 2002). Characterization of a member of the NnrR regulon in Rhodobacter sphaeroides 2.4.3 encoding a haem-copper protein: the GenBank accession number for nnrS is U62403. Microbiology 148, 825-833. |
| [7] | Baudouin E, Pieuchot L, Engler G, Pauly N, Puppo A ( 2006). Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol Plant Microbe Interact 19, 970-975. |
| [8] | Berger A, Brouquisse R, Pathak PK, Hichri I, Singh I, Bhatia S, Boscari A, Igamberdiev AU, Gupta KJ ( 2018). Pathways of nitric oxide metabolism and operation of phytoglobins in legume nodules: missing links and future directions. Plant Cell Environ 41, 2057-2068. |
| [9] | Bethke PC, Badger MR, Jones RL ( 2004). Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16, 332-341. |
| [10] | Blanquet P, Silva L, Catrice O, Bruand C, Carvalho H, Meilhoc E ( 2015). Sinorhizobium meliloti controls nitric oxide-mediated post-translational modification of a Medicago truncatula nodule protein. Mol Plant Microbe Interact 28, 1353-1363. |
| [11] | Boscari A, del Giudice J, Ferrarini A, Venturini L, Zaffini AL, Delledonne M, Puppo A ( 2013). Expression dynamics of the Medicago truncatula transcriptome during the symbiotic interaction with Sinorhizobium meliloti: which role for nitric oxide? Plant Physiol 161, 425-439. |
| [12] | Breakspear A, Liu CW, Roy S, Stacey N, Rogers C, Trick M, Morieri G, Mysore KS, Wen JQ, Oldroyd GED, Downie JA, Murray JD ( 2014). The root hair "infectome" of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 26, 4680-4701. |
| [13] | Calvo-Begueria L, Rubio MC, Martínez JI, Pérez-Ron- tomé C, Delgado MJ, Bedmar EJ, Becana M ( 2018). Redefining nitric oxide production in legume nodules through complementary insights from electron paramagnetic resonance spectroscopy and specific fluorescent probes. J Exp Bot 69, 3703-3714. |
| [14] | Cam Y, Pierre O, Boncompagni E, Hérouart D, Meilhoc E, Bruand C ( 2012). Nitric oxide (NO): a key player in the senescence of Medicago truncatula root nodules. New Phytol 196, 548-560. |
| [15] | Castillo MC, Lozano-Juste J, González-Guzmán M, Rodriguez L, Rodriguez PL, León J ( 2015). Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci Signal 8, ra89. |
| [16] | Chadha N, Mishra M, Rajpal K, Bajaj R, Choudhary DK, Varma A ( 2015). An ecological role of fungal endophytes to ameliorate plants under biotic stress. Arch Microbiol 197, 869-881. |
| [17] | Chaki M, Kovacs I, Spannagl M, Lindermayr C ( 2014). Computational prediction of candidate proteins for S-nitrosylation in Arabidopsis thaliana. PLoS One 9, e110232. |
| [18] | Cueto M, Hernández-Perera O, Martín R, Bentura ML, Rodrigo J, Lamas S, Golvano MP ( 1996). Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett 398, 159-164. |
| [19] | De Bruijn FJ, Rossbach S, Bruan C, Parrish JR ( 2006). A highly conserved Sinorhizobium meliloti operon is induced microaerobically via the FixLJ system and by nitric oxide (NO) via NnrR. Environ Microbiol 8, 1371-1381. |
| [20] | Dean JV, Harper JE ( 1988). The conversion of nitrite to nitrogen oxide(s) by the constitutive NAD(P)H-nitrate reductase enzyme from soybean. Plant Physiol 88, 389-395. |
| [21] | del Giudice J, Cam Y, Damiani I, Fung-Chat F, Meilhoc E, Bruand C, Brouquisse R, Puppo A, Boscari A ( 2011). Nitric oxide is required for an optimal establishment of the Medicago truncatula-Sinorhizobium meliloti symbiosis. New Phytol 191, 405-417. |
| [22] | Desalvo MK, Sunagawa S, Voolstra CR, Medina M ( 2010). Transcriptomic responses to heat stress and bleaching in the Elkhorn coral Acropora palmata. Mar Ecol Prog Ser 402, 97-113. |
| [23] | Ding YL, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, Harris JM, Oldroyd GED ( 2008). Abscisic acid coordinates nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 20, 2681-2695. |
| [24] | Escuredo PR, Minchin FR, Gogorcena Y, Iturbe-Or- maetxe I, Klucas RV, Becana M ( 1996). Involvement of activated oxygen in nitrate-induced senescence of pea root nodules. Plant Physiol 110, 1187-1195. |
| [25] | Ferrarini A, De Stefano M, Baudouin E, Pucciariello C, Polverari A, Puppo A, Delledonne M ( 2008). Expression of Medicago truncatula genes responsive to nitric oxide in pathogenic and symbiotic conditions. Mol Plant Microbe Interact 21, 781-790. |
| [26] | Garg N, Geetanjali , (2007). Symbiotic nitrogen fixation in legume nodules: process and signaling. A review. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C, eds. Sustainable Agriculture. Dordrecht: Springer. pp. 519-531. |
| [27] | Gonzalez-Rizzo S, Crespi M, Frugier F ( 2006). The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18, 2680-2693. |
| [28] | Gupta KJ, Hebelstrup KH, Mur LAJ, Igamberdiev AU ( 2011). Plant hemoglobins: important players at the crossroads between oxygen and nitric oxide. FEBS Lett 585, 3843-3849. |
| [29] | Hawkins TD, Krueger T, Becker S, Fisher PL, Davy SK ( 2014). Differential nitric oxide synthesis and host apoptotic events correlate with bleaching susceptibility in reef corals. Coral Reefs 33, 141-153. |
| [30] | Herold S, Puppo A ( 2005). Oxyleghemoglobin scavenges nitrogen monoxide and peroxynitrite: a possible role in functioning nodules? J Biol Inorg Chem 10, 935-945. |
| [31] | Hichri I, Boscari A, Castella C, Rovere M, Puppo A, Brouquisse R ( 2015). Nitric oxide: a multifaceted regulator of the nitrogen-fixing symbiosis. J Exp Bot 66, 2877-2887. |
| [32] | Hichri I, Boscari A, Meilhoc E, Catalá M, Barreno E, Bruand C, Lanfranco L, Brouquisse R (2016a). Nitric oxide: a multitask player in plant-microorganism symbioses. In: Lamattina L, García-Mata C, eds. Gasotransmitters in Plants: The Rise of a New Paradigm in Cell Signaling. Cham: Springer. pp. 239-268. |
| [33] | Hichri I, Meilhoc E, Boscari A, Bruand C, Frendo P, Brouquisse R ( 2016b). Nitric oxide: jack-of-all-trades of the nitrogen-fixing symbiosis? Adv Bot Res 77, 193-218. |
| [34] | Hill RD ( 2012). Non-symbiotic haemoglobins-what's happening beyond nitric oxide scavenging? AoB Plants 2012, pls004. |
| [35] | Horchani F, Prévot M, Boscari A, Evangelisti E, Meilhoc E, Bruand C, Raymond P, Boncompagni E, Aschi- Smiti S, Puppo A, Brouquisse R ( 2011). Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiol 15, 1023-1036. |
| [36] | Hu JL, Huang XH, Chen LC, Sun XW, Lu CM, Zhang LX, Wang YC, Zuo JR ( 2015). Site-speci?c nitrosoproteomic identi?cation of endogenously S-nitrosylated proteins in Arabidopsis. Plant Physiol 167, 1731-1746. |
| [37] | Iarullina DR, Asafova EV, Kartunova IE, Ziiatdinova GK, Il'inskaia ON ( 2014). Probiotics for plants: NO-producing lactobacilli protect plants from drought. Prikl Biokhim Mikrobiol 50, 189-192. |
| [38] | Igamberdiev AU, Hill RD ( 2004). Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55, 2473-2482. |
| [39] | Igamberdiev AU, Hill RD ( 2009). Plant mitochondrial function during anaerobiosis. Ann Bot 103, 259-268. |
| [40] | Igamberdiev AU, Ratcliffe RG, Gupta KJ ( 2014). Plant mitochondria: source and target for nitric oxide. Mitochondrion 19, 329-333. |
| [41] | Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, de Stefano M, Delledonne M, Puppo A, Baudouin E, Frendo P ( 2007). Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 225, 1597-1602. |
| [42] | Kato K, Kanahama K, Kanayama Y ( 2010). Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J Plant Physiol 167, 238-241. |
| [43] | Kearns EV, Assmann SM ( 1993). The guard cell-environment connection. Plant Physiol 102, 711-715. |
| [44] | Lee HW, Hitchcoc TM, Park SH, Mi R, Kraft JD, Luo J, Cao WG ( 2010). Involvement of thioredoxin domain-containing 5 in resistance to nitrosative stress. Free Radic Biol Med 49, 872-880. |
| [45] | Li BH, Li GJ, Kronzucker HJ, Balu?ka F, Shi WM ( 2014). Ammonium stress in Arabidopsis: signaling, genetic loci, and physiological targets. Trends Plant Sci 19, 107-114. |
| [46] | Lin AH, Wang YQ, Tang JY, Xue P, Li CL, Liu LC, Hu B, Yang FQ, Loake GJ, Chu CC ( 2012). Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol 158, 451-464. |
| [47] | Loscos J, Matamoros MA, Becana M ( 2008). Ascorbate and homoglutathione metabolism in common bean nodules under stress conditions and during natural senescence. Plant Physiol 146, 1282-1292. |
| [48] | Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M ( 1999). Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol 121, 879-888. |
| [49] | Matamoros MA, Saiz A, Pe?uelas M, Bustos-Sanmamed P, Mulet JM, Barja MV, Rouhier N, Moore M, James EK, Dietz KJ, Becana M ( 2015). Function of glutathione peroxidases in legume root nodules. J Exp Bot 66, 2979-2990. |
| [50] | Meakin GE, Bueno E, Jepson B, Bedmar EJ, Richardson DJ, Delgado MJ ( 2007). The contribution of bacteroidal nitrate and nitrite reduction to the formation of nitrosylleghaemoglobin complexes in soybean root nodules. Microbiology 153, 411-419. |
| [51] | Meilhoc E, Blanquet P, Cam Y, Bruand C ( 2013). Control of NO level in rhizobium-legume root nodules: not only a plant globin story. Plant Signal Behav 8, e25923. |
| [52] | Meilhoc E, Boscari A, Bruand C, Puppo A, Brouquisse R ( 2011). Nitric oxide in legume-rhizobium symbiosis. Plant Sci 181, 573-581. |
| [53] | Melo PM, Silva LS, Ribeiro I, Seabra AR, Carvalho HG ( 2011). Glutamine synthetase is a molecular target of nitric oxide in root nodules of Medicago truncatula and is regulated by tyrosine nitration. Plant Physiol 157, 1505-1517. |
| [54] | Moreau M, Lindermayr C, Durner J, Klessig DF ( 2010). NO synthesis and signaling in plants-where do we stand?. Physiol Plant 138, 372-383. |
| [55] | Mur LAJ, Prats E, Pierre S, Hall MA, Hebelstrup KH ( 2013). Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front Plant Sci 4, 215. |
| [56] | Murakami EI, Nagata M, Shimoda Y, Kucho KI, Higashi S, Abe M, Hashimoto M, Uchiumi T ( 2011). Nitric oxide production induced in roots of Lotus japonicus by lipopolysaccharide from Mesorhizobium loti. Plant Cell Physiol 52, 610-617. |
| [57] | Nagata M, Murakami EI, Shimoda Y, Shimoda-Sasakura F, Kucho KI, Suzuki A, Abe M, Higashi S, Uchiumi T ( 2008). Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microbe Interact 21, 1175-1183. |
| [58] | Navascués J, Pérez-Rontomé C, Gay M, Marcos M, Yang F, Walker FA, Desbois A, Abián J, Becana M ( 2012). Leghemoglobin green derivatives with nitrated hemes evidence production of highly reactive nitrogen species during aging of legume nodules. Proc Natl Acad Sci USA 109, 2660-2665. |
| [59] | Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I ( 2008). Nitric oxide, stomatal closure, and abiotic stress. J Exp Bot 59, 165-176. |
| [60] | Palmieri MC, Sell S, Huang X, Scherf M, Werner T, Durner J, Lindermayr C ( 2008). Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: a bioinformatics approach. J Exp Bot 59, 177-186. |
| [61] | Pérez-Chaca MV, Rodríguez-Serrano M, Molina AS, Pedranzani HE, Zirulnik F, Sandalio LM, Romero- Puertas MC ( 2014). Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ 37, 1672-1687. |
| [62] | Pérez Guerra JC, Coussens G, De Keyser A, De Rycke R, De Bodt S, Van De Velde W, Goormachtig S, Holsters M ( 2010). Comparison of developmental and stress-indu- ced nodule senescence in Medicago truncatula. Plant Physiol 152, 1574-1584. |
| [63] | Pii Y, Crimi M, Cremonese G, Spena A, Pandolfini T ( 2007). Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 7, 21. |
| [64] | Procházková D, Wilhelmová N ( 2011). Nitric oxide, reactive nitrogen species and associated enzymes during plant senescence. Nitric Oxide 24, 61-65. |
| [65] | Puppo A, Pauly N, Boscari A, Mandon K, Brouquisse R ( 2013). Hydrogen peroxide and nitric oxide: key regulators of the legume- Rhizobium and mycorrhizal symbioses. Antioxid Redox Signal 18, 2202-2219. |
| [66] | Radi R ( 2004). Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA 101, 4003-4008. |
| [67] | Romanov VI, Fedulova NG, Tchermenskaya IE, Shramko VI, Molchanov MI, Kretovich WL ( 1980). Metabolism of poly-hydroxybutyric acid in bacteroids of Rhizobium lupini in connection with nitrogen ?xation and photosynthesis. Plant Soil 56, 379-390. |
| [68] | Sainz M, Calvo-Begueria L, Pérez-Rontomé C, Wienkoop S, Abián J, Staudinger C, Bartesaghi S, Radi R, Becana M ( 2015). Leghemoglobin is nitrated in functional legume nodules in a tyrosine residue within the heme cavity by a nitrite/peroxide-dependent mechanism. Plant J 81, 723-735. |
| [69] | Sánchez C, Cabrera JJ, Gates AJ, Bedmar EJ, Richardson DJ, Delgado MJ ( 2011). Nitric oxide detoxification in the rhizobia-legume symbiosis. Biochem Soc Trans 39, 184-188. |
| [70] | Sánchez C, Gates AJ, Meakin GE, Uchiumi T, Girard L, Richardson DJ, Bedmar EJ, Delgado MJ ( 2010). Production of nitric oxide and nitrosylleghemoglobin complexes in soybean nodules in response to flooding. Mol Plant Microbe Interact 23, 702-711. |
| [71] | She XP, Song XG, He JM ( 2004). Role and relationship of nitric oxide and hydrogen peroxide in light/dark-regulated stomatal movement in Vicia faba. Acta Bot Sin 46, 1292-1300. |
| [72] | Shimoda Y, Nagata M, Suzuki A, Abe M, Sato S, Kato T, Tabata S, Higashi S, Uchiumi T ( 2005). Symbiotic rhizobium and nitric oxide induce gene expression of non- symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol 46, 99-107. |
| [73] | Singh VP, Singh S, Kumar J, Prasad SM ( 2015). Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: possible involvement of nitric oxide. J Plant Physiol 181, 20-29. |
| [74] | Smil V ( 1999). Detonator of the population explosion. Nature 400, 415. |
| [75] | Suzuki A, Akune M, Kogiso M, Imagama Y, Osuk K, Uchiumi T, Higashi S, Han SY, Yoshida S, Asami T, Abe M ( 2004). Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol 45, 914-922. |
| [76] | Swaraj K, Sheokand S, Fernandez-Pascual MM, de Felipe MR ( 2001). Dark-induced changes in legume nodule functioning. Aust J Plant Physiol 28, 429-438. |
| [77] | Tominaga A, Nagata M, Futsuki K, Abe H, Uchiumi T, Abe M, Kucho KI, Hashiguchi M, Akashi R, Hirsch A, Arima S, Suzuki A ( 2010). Effect of abscisic acid on symbiotic nitrogen fixation activity in the root nodules of Lotus japonicus. Plant Signal Behav 5, 440-443. |
| [78] | Trevaskis B, Watts RA, Andersson CR, Llewellyn DJ, Hargrove MS, Olson JS, Dennis ES, Peacock WJ ( 1997). Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA 94, 12230-12234. |
| [79] | Van de Velde W, Guerra JCP, De Keyser A, De Rycke R, Rombauts S, Maunoury N, Mergaert P, Kondorosi E, Holsters M, Goormachtig S ( 2006). Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol 141, 711-720. |
| [80] | Vinardell JM, Fedorova E, Cebolla A, Kevei Z, Horvath G, Kelemen Z, Tarayre S, Roudier F, Mergaert P, Kondorosi A, Kondorosi E ( 2003). Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15, 2093-2105. |
| [81] | Wally OSD, Mira MM, Hill RD, Stasolla C ( 2013). Hemoglobin regulation of plant embryogenesis and plant pathogen interaction. Plant Signal Behav 8, e25264. |
| [82] | Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F ( 2008). In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll ?uorescence in pea leaves. Plant Physiol 146, 1920-1927. |
| [83] | Yoshida T, Mogami J, Yamaguchi-Shinozaki K ( 2015). Omics approaches toward defining the comprehensive abscisic acid signaling network in plants. Plant Cell Physiol 56, 1043-1052. |
| [84] | Zeiger E ( 1983). The biology of stomatal guard cells. Annu Rev Plant Physiol 34, 441-474. |
| [85] | Zimmer-Prados LM, Moreira ASFP, Magalhaes JR, Fran?a MGC ( 2014). Nitric oxide increases tolerance responses to moderate water deficit in leaves of Phaseolus vulgaris and Vigna unguiculata bean species. Physiol Mol Biol Plants 20, 295-301. |
/
| 〈 |
|
〉 |