

Protocols for Analyzing Rice Meiotic Chromosomes
Received date: 2019-05-12
Accepted date: 2019-06-14
Online published: 2019-06-17
The development of techniques to analyze pachytene chromosomes has greatly overcome most of the difficulties in cytological studies of rice chromosomes caused by their small size. Visualization of meiotic chromosomes has now become routine in cytogenetic studies of this species. This chapter provides protocols on basic meiotic chromosome preparation, FISH analysis and immunocytology in rice.
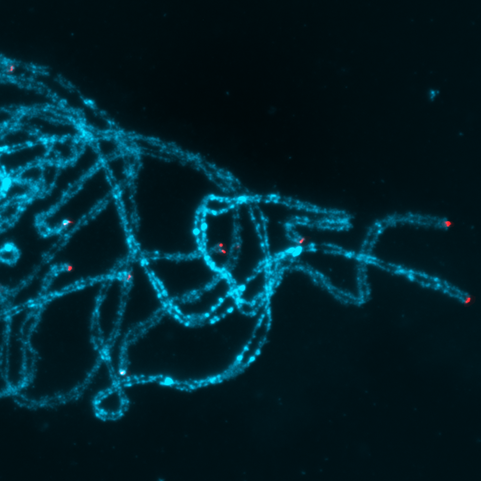
Key words: rice; meiosis; chromosome; FISH; immunostaining
Xinjie Cheng , Hengxiu Yu , Zhukuan Cheng . Protocols for Analyzing Rice Meiotic Chromosomes[J]. Chinese Bulletin of Botany, 2019 , 54(4) : 503 -508 . DOI: 10.11983/CBB19087
| [1] | Arumuganathan K, Earle ED ( 1991). Estimation of nuclear DNA content of plants by flow cytometry. Plant Mol Biol Rep 9, 229-241. |
| [2] | Che L, Tang D, Wang K, Wang M, Zhu K, Yu H, Gu M, Cheng Z ( 2011). OsAM1 is required for leptotene transition in rice. Cell Res 21, 654-665. |
| [3] | Cheng Z, Buell CR, Wing RA, Gu M, Jiang J ( 2001 a). Toward a cytological characterization of the rice genome. Genome Res 11, 2133-2141. |
| [4] | Cheng Z, Dong F, Langdon T, Ouyang S, Buell CR, Gu M, Blattner FR, Jiang J ( 2002). Functional rice centromeres are marked by a satellite repeat and a centromere-specific retrotransposon. Plant Cell 14, 1691-1704. |
| [5] | Cheng Z, Stupar RM, Gu M, Jiang J ( 2001 b). A tandemly repeated DNA sequence is associated with both knob-like heterochromatin and a highly decondensed structure in the meiotic pachytene chromosomes of rice. Chromosoma 110, 24-31. |
| [6] | Goff SA ( 1999). Rice as a model for cereal genomics. Curr Opin Plant Biol 2, 86-89. |
| [7] | Ji JH, Tang D, Shen Y, Xue ZH, Wang HJ, Shi WQ, Zhang C, Du GJ, Li YF, Cheng ZK ( 2016). P31 comet, a member of the synaptonemal complex, participates in meiotic DSB formation in rice . Proc Natl Acad Sci USA 113, 10577-10582. |
| [8] | Kurata N, Omura T, Iwata N ( 1981). Studies on centromere, chromomere and nucleolus in pachytene nuclei of rice, Oryza sativa, microsporocytes. Cytologia 46, 791-800. |
| [9] | Li X, Chao D, Wu Y, Huang X, Chen K, Cui L, Su L, Ye W, Chen H, Chen H, Dong N, Guo T, Shi M, Feng Q, Zhang P, Han B, Shan J, Gao J, Lin H ( 2015). Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet 47, 827-833. |
| [10] | Li YF, Qin BX, Shen Y, Zhang FF, Liu CZ, You HL, Du GJ, Tang D, Cheng ZK ( 2018). HEIP1 regulates crossover formation during meiosis in rice. Proc Natl Acad Sci USA 115, 10810-10815. |
| [11] | Luo Q, Li YF, Shen Y, Cheng ZK ( 2014). Ten years of gene discovery for meiotic event control in rice. J Genet Genomics 41, 125-137. |
| [12] | Ma Y, Dai X, Xu Y, Luo W, Zheng X, Zeng D, Pan Y, Lin X, Liu H, Zhang D, Xiao J, Guo X, Xu S, Niu Y, Jin J, Zhang H, Xu X, Li L, Wang W, Qian Q, Ge S, Chong K ( 2015). COLD1 confers chilling tolerance in rice. Cell 160, 1209-1221. |
| [13] | Miao CB, Tang D, Zhang HG, Wang M, Li YF, Tang SZ, Yu HX, Gu MH, Cheng ZK ( 2013). CENTRAL REGION COMPONENT 1, a novel synaptonemal complex component, is essential for meiotic recombination initiation in rice. Plant Cell 25, 2998-3009. |
| [14] | Nonomura K, Morohoshi A, Nakano M, Eiguchi M, Miyao A, Hirochika H, Kurata N ( 2007). A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell 19, 2583-2594. |
| [15] | Pawlowski WP, Golubovskaya IN, Timofejeva L, Meeley RB, Sheridan WF, Cande WZ ( 2004). Coordination of meiotic recombination, pairing, and synapsis by PHS1. Science 303, 89-92. |
| [16] | Ren LJ, Tang D, Zhao TT, Zhang FF, Liu CZ, Xue ZH, Shi WQ, Du GJ, Shen Y, Li YF, Cheng ZK ( 2018). OsSPL regulates meiotic fate acquisition in rice. New Phytol 218, 789-803. |
| [17] | Tang X, Bao W, Zhang W, Cheng Z ( 2007). Identification of chromosomes from multiple rice genomes using a universal molecular cytogenetic marker system. J Integr Plant Biol 49, 953-960. |
| [18] | Wang K, Tang D, Wang M, Lu J, Yu H, Liu J, Qian B, Gong Z, Wang X, Chen J, Gu M, Cheng Z ( 2009). MER3 is required for normal meiotic crossover formation, but not for presynaptic alignment in rice. J Cell Sci 122, 2055-2063. |
| [19] | Wang M, Wang K, Tang D, Wei C, Li M, Shen Y, Chi Z, Gu M, Cheng Z ( 2010). The central element protein ZEP1 of the synaptonemal complex regulates the number of crossovers during meiosis in rice. Plant Cell 22, 417-430. |
| [20] | Wang Y, Xiong G, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L, Xu E, Xu J, Ye W, Meng X, Liu R, Chen H, Jing Y, Wang Y, Zhu X, Li J, Qian Q ( 2015). Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet 47, 944-948. |
| [21] | Wu HK ( 1967). Note on preparing of pachytene chromosomes by double mordant. Sci Agric 15, 40-44. |
| [22] | Yu H, Wang M, Tang D, Wang K, Chen F, Gong Z, Gu M, Cheng Z ( 2010). OsSPO11-1 is essential for both homologous chromosome pairing and crossover formation in rice. Chromosoma 119, 625-636. |
| [23] | Zhang F, Tang D, Shen Y, Xue ZH, Shi WQ, Ren LJ, Du GJ, Li Y, Cheng ZK ( 2017). The F-box protein ZYGO1 mediates bouquet formation to promote homologous pairing, synapsis, and recombination in rice meiosis. Plant Cell 29, 2597-2609. |
| [24] | Zhao TT, Ren LJ, Chen XJ, Yu HX, Liu CJ, Shen Y, Shi WQ, Tang D, Du GJ, Li YF, Ma BJ, Cheng ZK ( 2018). The OsRR24/LEPTO1 type-B response regulator is essential for the organization of leptotene chromosomes in rice meiosis. Plant Cell 30, 3024-3037. |
/
| 〈 |
|
〉 |