

Expression Analysis of β-1,3-Glucanase Gene from Winter Brassica rapa Under Low Temperature Stress
Received date: 2016-11-23
Accepted date: 2017-03-24
Online published: 2017-07-10
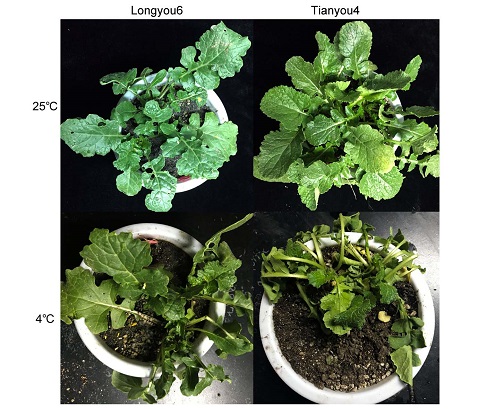
This study examined the function of β-1,3-glucanase in Brassica rapa with low temperature stress, whose encoding protein was identified by protein mass spectrometry analysis. The cDNA sequence of β-1,3-glucanase was cloned by RT-PCR of winter rapeseed from Longyou6 and Tianyou4 cultivars. Semi-quantitative PCR and real-time fluorescence quantitative PCR were used to study the expression pattern of β-1,3-glucanase in response to low temperature stress. The open reading frame of the β-1,3-glucanase gene was obtained at a length of 1 032 bp, and encoded 343 amino acid. The molecular weight was 38.102 kDa and the isoelectric point 6.63, with 93.94% amino sequence similarity to B. rapa subsp. chinensis and B. napus. The protein encoded by this gene is a hydrophilic protein with one signal peptide and two transmembrane domains. The prediction of the secondary structures indicated that β-1,3-glucanase is a protein with more α-helices. The predicted β-1,3-glucanase contains a conserved amino acid sequence corresponding to the plant Glycol-Hydro 17 superfamily. RT-PCR and semi-quantitative RT-PCR results showed that β-1,3-glucanase was upregulated in response to 4°C, and the gene was upregulated to peak in expression at -4°C; However, the expression was inhibited at lower temperature (-8°C). The β-1,3-glucanase gene cloned from the winter B. rapa cv. ‘Longyou6’ might play a role in cold tolerance.
Key words: β-1; 3-glucanase gene; low temperature; molecular cloning; expression analysis
Li Ma, Wancang Sun, Jinhai Yuan, Zigang Liu, Junyan Wu, Yan Fang, Yaozhao Xu, Yuanyuan Pu, Jing Bai, Xiaoyun Dong, Huili He . Expression Analysis of β-1,3-Glucanase Gene from Winter Brassica rapa Under Low Temperature Stress[J]. Chinese Bulletin of Botany, 2017 , 52(5) : 568 -578 . DOI: 10.11983/CBB16225
| [1] | 陈芳兰, 林玉玲, 陈裕坤, 冯新, 张梓浩, 赖钟雄 (2015). 三明野生蕉β-1,3-葡聚糖酶基因克隆及其低温下SA处理后的表达分析. 西北植物学报 35, 1709-1721. |
| [2] | 程红梅, 简桂良, 倪万潮, 杨红华, 王志兴, 孙文姬, 张保龙, 王晓峰, 马存, 贾士荣 (2005). 转几丁质酶和β-1,3-葡聚糖酶基因提高棉花对枯萎病和黄萎病的抗性. 中国农业科学 38, 1160-1166. |
| [3] | 邓文星, 张映 (2007). 实时荧光定量PCR技术综述. 生物技术通报(5), 93-95, 103. |
| [4] | 高玉龙, 郭旺珍, 王磊, 张天真 (2007). 一个棉花β-1,3-葡聚糖酶基因全长cDNA的克隆与特征分析. 作物学报 33, 1310-1315. |
| [5] | 龚束芳, 杨涛, 车代弟 (2010). 抗冻蛋白溶液中冰晶生长行为的研究. 上海交通大学学报(农业科学版) 28, 265-268, 279. |
| [6] | 郭尧君 (2005). 蛋白质电泳实验技术(第2版). 北京: 科学出版社. pp. 123-156. |
| [7] | 何江峰, 韩冰, 郭慧琴, 张占雄, 赵雅丽 (2007). 燕麦β-1,3-葡聚糖酶II基因3′端cDNA的克隆及分析. 生物技术 17(1), 5-8. |
| [8] | 何江峰, 韩冰, 赵宏鑫 (2006). 植物β-1,3-葡聚糖酶的研究进展. 内蒙古农业科技 5, 21-25. |
| [9] | 蒋选利, 李振岐, 康振生 (2005). β-1,3-葡聚糖酶与植物的抗病性. 西北农业学报 14, 135-139. |
| [10] | 蓝海燕, 王长海, 张丽华, 刘桂珍, 王岚兰, 陈正华, 田颖川 (2000). 导入β-1,3-葡聚糖酶及几丁质酶基因的转基因可育油菜及其抗菌核病的研究. 生物工程学报 16, 142-146. |
| [11] | 林植芳, 刘楠 (2012). 活性氧调控植物生长发育的研究进展. 植物学报 47, 74-86. |
| [12] | 马文月 (2004). 植物冷害和抗冷性的研究进展. 安徽农业科学 32, 1003-1006. |
| [13] | 欧阳波, 李汉霞, 叶志彪 (2002). 植物β-1,3-葡聚糖酶及其基因. 中国生物工程杂志 22, 18-23. |
| [14] | 孙万仓, 马卫国, 雷建民, 刘秦, 杨仁义, 武军艳, 王学芳, 叶剑, 曾军, 张亚宏, 康艳丽, 郭秀娟, 魏文惠, 杨杰, 蒲媛媛, 曾潮武, 刘红霞 (2007). 冬油菜在西北旱寒区的适应性和北移的可行性研究. 中国农业科学 40, 2716-2726. |
| [15] | 孙万仓, 刘自刚, 周冬梅, 张仁陟 (2016). 北方冬油菜北移与区划. 北京: 科学出版社. pp. 45-47. |
| [16] | 杨刚, 史鹏辉, 孙万仓, 刘自刚, 曾秀存, 武军艳, 方彦, 李学才, 陈奇, 刘林波, 杨建胜, 方园, 张娟 (2016). 白菜型冬油菜质外体抗冻蛋白研究. 中国生态农业学报 24, 210-217. |
| [17] | 翟国会, 阮小蕾, 吴丽婷, 谭小勇, 李华平 (2011). 指天蕉β-1,3-葡聚糖酶基因全长cDNA的克隆及序列分析. 中国农业科学 44, 3134-3141. |
| [18] | 张妙霞 (2010). 野生香蕉(Musa spp., AB group)抗寒相关基因的克隆与表达分析. 博士论文. 福州: 福建农林大学. pp. 134-140. |
| [19] | 赵娟, 兰海燕 (2011). 拟南芥β-1,3-葡聚糖酶基因(BG1)在不同组织及非生物胁迫下的表达研究. 新疆农业科学 48, 712-718. |
| [20] | 周丽英, 杨丽涛, 郑坚瑜 (2001). 植物抗寒冻基因工程研究进展. 植物学通报 18, 325-331. |
| [21] | Beffa R, Meins Jr FM (1996). Pathogenesis-related function of plant β-1,3-glucanae investigated by antisense transformation—a review.Gene 179, 97-103. |
| [22] | Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical Biochem 72, 248-254. |
| [23] | Griffith M, Ala P, Yang DSC, Hon WC, Moffatt BA (1992). Antifreeze protein produced endogenously in winter rye leaves.Plant Physiol 100, 593-596. |
| [24] | Hon WC, Griffith M, Chong P, Yang DSC (1994). Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves.Plant Physiol 104, 971-980. |
| [25] | Masoud SA, Zhu Q, Lamb C, Dixon RA (1996). Constitutive expression of an inducible β-1,3-glucanase in alfalfa reduces disease severity caused by the oomycete patho- gen Phytophthora megasperma f. spmedicaginis, but does not reduce disease severity of chitin-containing fungi.Transgenic Res 5, 313-323. |
| [26] | Mezabasso L, Alberdi M, Raynal M, Ferrerocadinanos ML, Delseny M (1986). Changes in protein synthesis in rapeseed (Brassica napus) seedlings during a low temperature treatment.Plant Physiol 82, 733-738. |
| [27] | Nakamura Y, Sawada H, Kobayashi S, Nakajima I, Yoshikawa M (1999). Expression of soybean β-1,3-endo- glucanase cDNA and effect on disease tolerance in kiwifruit plants.Plant Cell Rep 18, 527-532. |
| [28] | Xiong LM, Zhu JK (2001). Abiotic stress signal transduction in plants: molecular and genetic perspectives.Physiol Plant 112, 152-166. |
| [29] | Zhu Q, Maher EA, Masoud S, Dixon RA, Lamb CJ (1994). Enhanced protection against fungal attack by constitutive co-expression of chitinase and glucanase genes in trans- genic tobacco.Biol Technol 12, 807-812. |
/
| 〈 |
|
〉 |