

Received date: 2016-06-07
Accepted date: 2016-11-11
Online published: 2017-07-10
The cuticle is assembled at the surface of plant epidermal cells as a hydrophobic coating. It acts as an efficient barrier that protects the plant against uncontrolled water loss as well as environmental stress. It also plays a role in self-cleaning and plant development. The cuticle mainly consists of wax and cutin. The major structural component of the cuticle is cutin, which is a polyester rich in oxygenated fatty acids and glycerol. The cuticular waxes are complex mixtures of hydrophobic material containing predominantly very-long-chain fatty acids and their derivatives. The biosynthesis of polyester monomers as well as aliphatic wax components are localized at the endoplasmic reticulum. Then they are transported to the surface of plant epidermal cells and assembled into a functional cuticle structure. Much progress has been made in understanding the steps of biosynthesis, transport, formation and regulation of cuticular components by study of cuticle genes. The pathways of wax and cutin synthesis are gradually emerging due to the advances of cuticle gene-related research. The mapping and functional analysis of the ABCG full transporter has been a breakthrough in cuticle secretion research. A deeper understanding of the formation of cuticle layers has been achieved with the analysis of esterase and lipase-related cuticle genes. In terms of regulation, the findings of transcription factor genes, as well as the interaction mechanism between cuticle and the environment, have increased our knowledge of regulatory circuits. We review the current progress in study of these important genes.
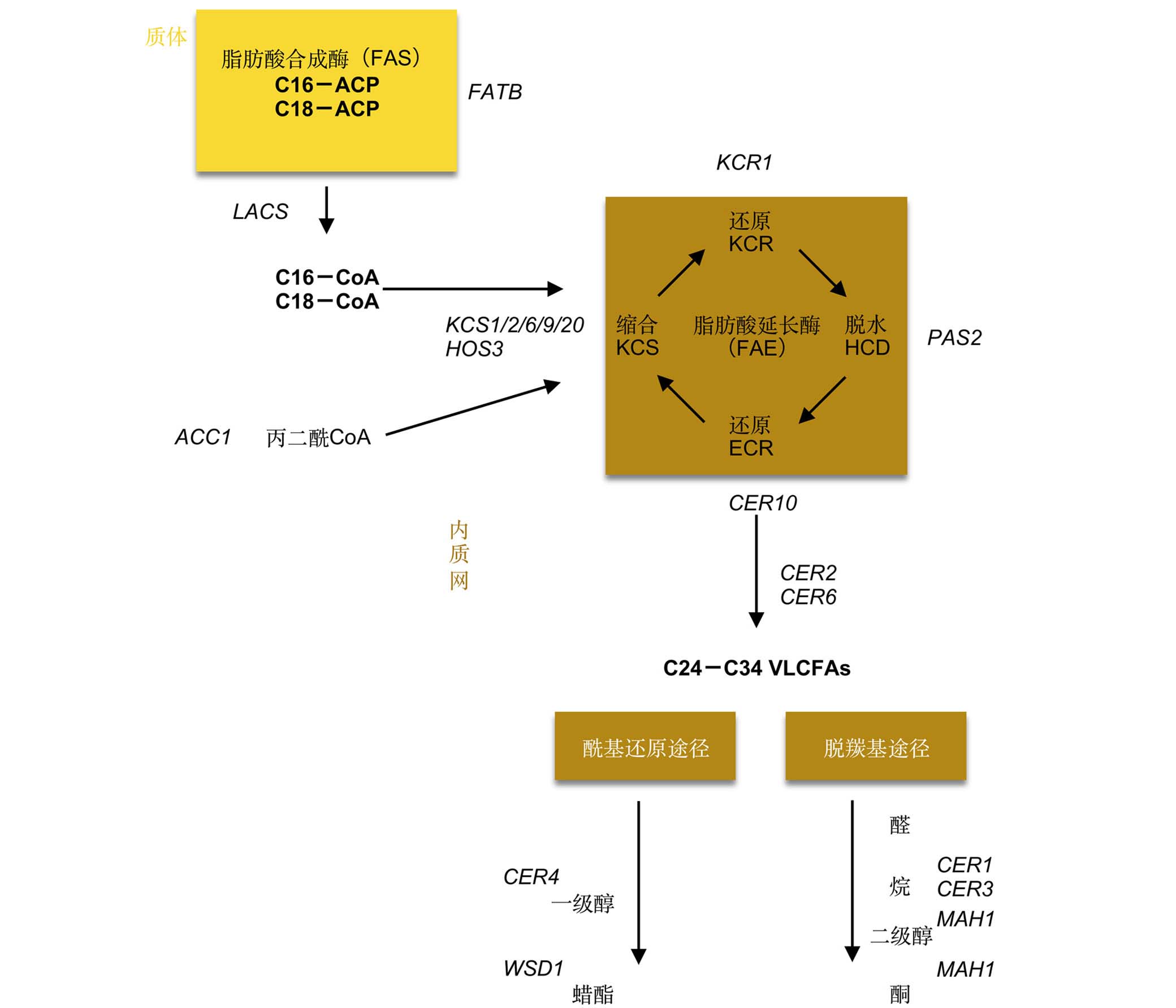
Key words: cuticle; biosynthesis; transport; formation; gene
Aidong Wang , Guoxiong Chen , Ruijun Duan . Advances in Study of Plant Cuticle Genes[J]. Chinese Bulletin of Botany, 2017 , 52(5) : 637 -651 . DOI: 10.11983/CBB16127
| [1] | Aarts MG, Keijzer CJ, Stiekema WJ, Pereira A (1995). Molecular characterization of the CER1 gene of Arabi- dopsis involved in epicuticular wax biosynthesis and pollen fertility.Plant Cell 7, 2115-2127. |
| [2] | Achyuthan KE, Achyuthan AM, Adams PD, Dirk SM, Harper JC, Simmons BA, Singh AK (2010). Supramole- cular self-assembled chaos: polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels.Molecules 15, 8641-8688. |
| [3] | Aharoni A, Dixit S, Jetter R, Thoenes E, Van Arkel G, Pereira A (2004). The SHINE clade of AP2 domain trans- cription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overex- pressed in Arabidopsis.Plant Cell 16, 2463-2480. |
| [4] | Bach L, Michaelson LV, Haslam R, Bellec Y, Gissot L, Marion J, Da Costa M, Boutin JP, Miquel M, Tellier F, Domergue F, Markham JE, Beaudoin F, Napier JA, Faure JD (2008). The very-long-chain hydroxy fatty acyl- CoA dehydratase PASTICCINO2 is essential and limi- ting for plant development.Proc Natl Acad Sci USA 105, 14727-14731. |
| [5] | Barthlott W, Neinhuis C (1997). Purity of the sacred lotus, or escape from contamination in biological surfaces.Planta 202, 1-8. |
| [6] | Bateman RM, Crane PR, DiMichele WA, Kenrick PR, Rowe NP, Speck T, Stein WE (1998). Early evolution of land plants: phylogeny, physiology, and ecology of the primary terrestrial radiation.Annu Rev Ecol Syst 29, 263-292. |
| [7] | Beaudoin F, Wu XZ, Li FL, Haslam RP, Markham JE, Zheng HQ, Napier JA, Kunst L (2009). Functional characterization of the Arabidopsis β-ketoacyl-coenzyme A reductase candidates of the fatty acid elongase.Plant Phy- siol 150, 1174-1191. |
| [8] | Beisson F, Li-Beisson Y, Pollard M (2012). Solving the puzzles of cutin and suberin polymer biosynthesis.Curr Opin Plant Biol 15, 329-337. |
| [9] | Bernard A, Domergue F, Pascal S, Jetter R, Renne C, Faure JD, Haslam RP, Napier JA, Lessire R, Joubès J (2012). Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECE- RIFERUM3 are core components of a very-long-chain alk- ane synthesis complex.Plant Cell 24, 3106-3118. |
| [10] | Bessire M, Borel S, Fabre G, Carraça L, Efremova N, Yephremov A, Cao Y, Jetter R, Jacquat AC, Métraux JP, Nawrath C (2011). A member of the PLEIOTROPIC DRUG RESISTANCE family of ATP binding cassette trans- porters is required for the formation of a functional cuticle in Arabidopsis.Plant Cell 23, 1958-1970. |
| [11] | Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, Petétot JMC, Métraux JP, Nawrath C (2007). A permea- ble cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea.EMBO J 26, 2158-2168. |
| [12] | Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu XM, Yephremov A, Samuels L (2007). Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuti- cular lipid secretion.Plant J 52, 485-498. |
| [13] | Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003). Disruption of the FATB gene in Arabidopsis de- monstrates an essential role of saturated fatty acids in plant growth.Plant Cell 15, 1020-1033. |
| [14] | Chen GX, Komatsuda T, Ma JF, Li C, Yamaji N, Nevo E (2011a). A functional cutin matrix is required for plant protection against water loss.Plant Signal Behav 6, 1297-1299. |
| [15] | Chen GX, Komatsuda T, Ma JF, Nawrath C, Pourkheir- andish M, Tagiri A, Hu YG, Sameri M, Li XR, Zhao X, Liu YB, Li C, Ma XY, Wang AD, Nair S, Wang N, Miyao A, Sakuma S, Yamaji N, Zheng XT, Nevo E (2011b). An ATP-binding cassette subfamily G full transporter is ess- ential for the retention of leaf water in both wild barley and rice.Proc Natl Acad Sci USA 108, 12354-12359. |
| [16] | Chen GX, Komatsuda T, Pourkheirandish M, Sameri M, Sato K, Krugman T, Fahima T, Korol AB, Nevo E (2009). Mapping of the eibi1 gene responsible for the drought hypersensitive cuticle in wild barley (Hordeum spon- taneum).Breed Sci 59, 21-26. |
| [17] | Chen XB, Goodwin SM, Boroff VL, Liu XL, Jenks MA (2003). Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax pro- duction.Plant Cell 15, 1170-1185. |
| [18] | Chen XB, Goodwin SM, Liu XL, Chen XL, Bressan RA, Jenks MA (2005). Mutation of the RESURRECTION1 locus of Arabidopsis reveals an association of cuticular wax with embryo development.Plant Physiol 139, 909-919. |
| [19] | Cho BK, Kim MS, Baek IS, Kim DY, Lee WH, Kim J, Bae H, Kim YS (2013). Detection of cuticle defects on cherry tomatoes using hyperspectral fluorescence imagery.Post- harvest Biol Technol 76, 40-49. |
| [20] | Cominelli E, Sala T, Calvi D, Gusmaroli G, Tonelli C (2008). Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability.Plant J 53, 53-64. |
| [21] | Croteau R, Kolattukudy PE (1975). Biosynthesis of hydroxy- fatty acid polymers: enzymatic hydration of 18-hydroxy- cis-9,10-epoxystearic acid to threo-9,10,18-trihydroxyst- earic acid by a particulate preparation from apple (Malus pumila).Arch Biochem Biophys 170, 73-81. |
| [22] | DeBono A, Yeats TH, Rose JKC, Bird D, Jetter R, Kunst L, Samuels L (2009). Arabidopsis LTPG is a glycosylpho- sphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface.Plant Cell 21, 1230-1238. |
| [23] | Duan RJ, Xiong HY, Wang AD, Chen GX (2015). Molecular mechanisms underlying hull-caryopsis adhesion/separa- tion revealed by comparative transcriptomic analysis of co- vered/naked barley (Hordeum vulgare L.).Inter J Mol Sci 16, 14181-14193. |
| [24] | Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L (2010). MYB transcription factors in Ara- bidopsis.Trends Plant Sci 15, 573-581. |
| [25] | Dunn TM, Lynch DV, Michaelson LV, Napier JA (2004). A post-genomic approach to understanding sphingolipid me- tabolism in Arabidopsis thaliana.Ann Bot 93, 483-497. |
| [26] | Fich EA, Segerson NA, Rose JKC (2016). The plant poly- ester cutin: biosynthesis, structure, and biological roles.Ann Rev Plant Biol 67, 207-233. |
| [27] | Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D (2000). Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems.Plant Cell 12, 2001-2008. |
| [28] | Franke R, Briesen I, Wojciechowski T, Faust A, Yephre- mov A, Nawrath C, Schreiber L (2005). Apoplastic poly- esters in Arabidopsis surface tissues—a typical suberin and a particular cutin.Phytochemistry 66, 2643-2658. |
| [29] | Franke R, Höfer R, Briesen I, Emsermann M, Efremova N, Yephremov A, Schreiber L (2009). The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds.Plant J 57, 80-95. |
| [30] | Franke R, Schreiber L (2007). Suberin—a biopolyester forming apoplastic plant interfaces.Curr Opin Plant Biol 10, 252-259. |
| [31] | Gabarayeva NI, Grigorjeva VV (2013). Experimental model- ling of exine-like structures.Grana 52, 241-257. |
| [32] | Garroum I, Bidzinski P, Daraspe J, Mucciolo A, Humbel BM, Morel JB, Nawrath C (2016). Cuticular defects in Oryza sativa ATP-binding cassette transporter G31 mutant plants cause dwarfism, elevated defense responses and pathogen resistance.Plant Cell Physiol 57, 1179-1188. |
| [33] | Girard AL, Mounet F, Lemaire-Chamley M, Gaillard C, Elmorjani K, Vivancos J, Runavot JL, Quemener B, Petit J, Germain V, Rothan C, Marion D, Bakan B (2012). Tomato GDSL1 is required for cutin deposition in the fruit cuticle.Plant Cell 24, 3119-3134. |
| [34] | Go YS, Kim H, Kim HJ, Suh MC (2014). Arabidopsis cuti- cular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-type transcription factor.Plant Cell 26, 1666-1680. |
| [35] | Goodwin SM, Jenks MA (2005). Plant cuticle function as a barrier to water loss. In: Jenks MA, Hasegawa PM, eds. Plant Abiotic Stress. Oxford: Wiley-Blackwell. pp. 14-36. |
| [36] | Greer S, Wen M, Bird D, Wu XM, Samuels L, Kunst L, Jetter R (2007). The cytochrome P450 enzyme CYP- 96A15 is the midchain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuti- cular wax of Arabidopsis.Plant Physiol 145, 653-667. |
| [37] | Han JX, Clement JM, Li J, King A, Ng S, Jaworski JG (2010). The cytochrome P450 CYP86A22 is a fatty acyl- CoA ω-hydroxylase essential for estolide synthesis in the stigma of Petunia hybrida.J Biol Chem 285, 3986-3996. |
| [38] | Haslam TM, Mañas-Fernández A, Zhao LF, Kunst L (2012). Arabidopsis ECERIFERUM2 is a component of the fatty acid elongation machinery required for fatty acid exten- sion to exceptional lengths.Plant Physiol 160, 1164-1174. |
| [39] | Heredia-Guerrero JA, Benítez JJ, Heredia A (2008). Self- assembled polyhydroxy fatty acids vesicles: a mechanism for plant cutin synthesis.Bioessays 30, 273-277. |
| [40] | Hooker TS, Lam P, Zheng HQ, Kunst L (2007). A core subunit of the RNA-processing/degrading exosome speci- fically influences cuticular wax biosynthesis in Arabi- dopsis.Plant Cell 19, 904-913. |
| [41] | Hooker TS, Millar AA, Kunst L (2002). Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis.Plant Physiol 129, 1568-1580. |
| [42] | Jetter R, Kunst L, Samuels AL (2006). Composition of plant cuticular waxes. In: Riederer M, Müller C, eds. Annual Plant Reviews. Vol. 23. Oxford: Blackwell. pp. 145-181. |
| [43] | Joubès J, Raffaele S, Bourdenx B, Garcia C, Laroche- Traineau J, Moreau P, Domergue F, Lessire R (2008). The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression pro- filing.Plant Mol Biol 67, 547-566. |
| [44] | Kannangara R, Branigan C, Liu Y, Penfield T, Rao V, Mouille G, Höfte H, Pauly M, Riechmann JL, Broun P (2007). The transcription factor WIN1/SHN1 regulates cu- tin biosynthesis in Arabidopsis thaliana.Plant Cell 19, 1278-1294. |
| [45] | Kerstiens G (1996). Cuticular water permeability and its physiological significance.J Exp Bot 47, 1813-1832. |
| [46] | Kim J, Jung JH, Lee SB, Go YS, Kim HJ, Cahoon R, Markham JE, Cahoon EB, Suh MC (2013). Arabidopsis 3-ketoacyl-coenzyme A synthase9 is involved in the syn- thesis of tetracosanoic acids as precursors of cuticular waxes, suberins, sphingolipids, and phospholipids.Plant Physiol 162, 567-580. |
| [47] | Kosma DK, Bourdenx B, Bernard A, Parsons EP, Lü SY, Joubès J, Jenks MA (2009). The impact of water defi- ciency on leaf cuticle lipids of Arabidopsis.Plant Physiol 151, 1918-1929. |
| [48] | Kunst L, Samuels AL (2003). Biosynthesis and secretion of plant cuticular wax.Prog Lipid Res 42, 51-80. |
| [49] | Kunst L, Samuels L (2009). Plant cuticles shine: advances in wax biosynthesis and export.Curr Opin Plant Biol 12, 721-727. |
| [50] | Kunst L, Taylor DC, Underhill EW (1992). Fatty acid elon- gation in developing seeds of Arabidopsis thaliana.Plant Physiol Biochem 30, 425-434. |
| [51] | Kurdyukov S, Faust A, Nawrath C, Bär S, Voisin D, Efremova N, Franke R, Schreiber L, Saedler H, Métraux JP, Yephremov A (2006a). The epidermis-specific extrace- llular BODYGUARD controls cuticle development and mor- phogenesis in Arabidopsis.Plant Cell 18, 321-339. |
| [52] | Kurdyukov S, Faust A, Trenkamp S, Bär S, Franke R, Efremova N, Tietjen K, Schreiber L, Saedler H, Yephre- mov A (2006b). Genetic and biochemical evidence for involvement of HOTHEAD in the biosynthesis of long- chain α-, ω-dicarboxylic fatty acids and formation of extra- cellular matrix.Planta 224, 315-329. |
| [53] | Lam P, Zhao LF, McFarlane HE, Aiga M, Lam V, Hooker TS, Kunst L (2012). RDR1 and SGS3, components of RNA-mediated gene silencing, are required for the regu- lation of cuticular wax biosynthesis in developing inflore- scence stems of Arabidopsis.Plant Physiol 159, 1385-1395. |
| [54] | Lee SB, Go YS, Bae HJ, Park JH, Cho SH, Cho HJ, Lee DS, Park OK, Hwang I, Suh MC (2009). Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plasto- globules, and enhanced susceptibility to infection by the fungal pathogen Alternaria brassicicola.Plant Physiol 150, 42-54. |
| [55] | Lee SB, Kim H, Kim RJ, Suh MC (2014). Overexpression of Arabidopsis MYB96 confers drought resistance in Came- lina sativa via cuticular wax accumulation.Plant Cell Rep 33, 1535-1546. |
| [56] | Lee SB, Suh MC (2013). Recent advances in cuticular wax biosynthesis and its regulation in Arabidopsis.Mol Plant 6, 246-249. |
| [57] | Lee SB, Suh MC (2015). Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species.Plant Cell Rep 34, 557-572. |
| [58] | L'haridon F, Besson-Bard A, Binda M, Serrano M, Abou- Mansour E, Balet F, Schoonbeek HJ, Hess S, Mir R, Léon J, Lamotte O, Métraux JP (2011). A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity.PLoS Pathog 7, e1002148. |
| [59] | Li C, Wang AD, Ma XY, Nevo E, Chen GX (2010). Con- sensus maps of cloned plant cuticle genes.Sci Cold Arid Regions 2, 465-476. |
| [60] | Li FL, Wu XM, Lam P, Bird D, Zheng HQ, Samuels L, Jetter R, Kunst L (2008). Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabi- dopsis.Plant Physiol 148, 97-107. |
| [61] | Li YH, Beisson F, Koo AJK, Molina I, Pollard M, Ohlrogge J (2007). Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers.Proc Natl Acad Sci USA 104, 18339-18344. |
| [62] | Li-Beisson Y, Pollard M, Sauveplane V, Pinot F, Ohlrogge J, Beisson F (2009). Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester.Proc Natl Acad Sci USA 106, 22008-22013. |
| [63] | Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, DeBono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu CC, Zallot R, Ohlrogge J (2010). Acyl-lipid metabo- lism.Arabidopsis Book 8, e0133. |
| [64] | Lippold F, Sanchez DH, Musialak M, Schlereth A, Sch- eible WR, Hincha DK, Udvardi MK (2009). AtMyb41 regulates transcriptional and metabolic responses to osmotic stress in Arabidopsis.Plant Physiol 149, 1761-1772. |
| [65] | Lolle SJ, Pruitt RE (1999). Epidermal cell interactions: a case for local talk.Trends Plant Sci 4, 14-20. |
| [66] | Lü SY, Song T, Kosma DK, Parsons EP, Rowland O, Jen- ks MA (2009). Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlap- ping functions with LACS2 in plant wax and cutin synthe- sis.Plant J 59, 553-564. |
| [67] | Lü SY, Zhao HY, Des Marais DL, Parsons EP, Wen XX, Xu XJ, Bangarusamy DK, Wang GC, Rowland O, Juenger T (2012). Arabidopsis ECERIFERUM9 involvement in cuticle formation and maintenance of plant water status.Plant Physiol 159, 930-944. |
| [68] | Lü SY, Zhao HY, Parsons EP, Xu CC, Kosma DK, Xu XJ, Chao DY, Lohrey G, Bangarusamy DK, Wang GC (2011). The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by Arabidopsis.Plant Phy- siol 157, 1079-1092. |
| [69] | Luo B, Xue XY, Hu WL, Wang LJ, Chen XY (2007). An ABC transporter gene of Arabidopsis thaliana, AtWBC11, is involved in cuticle development and prevention of organ fusion.Plant Cell Physiol 48, 1790-1802. |
| [70] | Ma XY, Li C, Wang AD, Duan RJ, Jiao GL, Nevo E, Chen GX (2012a). Genetic diversity of wild barley (Hordeum vulgare ssp. spontaneum) and its utilization for barley improvement.Sci Cold Arid Regions 4, 453-461. |
| [71] | Ma XY, Sela H, Jiao GL, Li C, Wang AD, Pourkheirandish M, Weiner D, Sakuma S, Krugman T, Nevo E, Koma- tsuda T, Korol A, Chen GX (2012b). Population-genetic analysis of HvABCG31 promoter sequence in wild barley (Hordeum vulgare ssp. spontaneum).BMC Evol Biol 12, 188. |
| [72] | McFarlane HE, Shin JJH, Bird DA, Samuels AL (2010). Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different com- binations.Plant Cell 22, 3066-3075. |
| [73] | McFarlane HE, Watanabe Y, Yang WL, Huang Y, Ohlrogge J, Samuels AL (2014). Golgi- and trans-Golgi network- mediated vesicle trafficking is required for wax secretion from epidermal cells.Plant Physiol 164, 1250-1260. |
| [74] | Ménard R, Verdier G, Ors M, Erhardt M, Beisson F, Shen WH (2014). Histone H2B monoubiquitination is involved in the regulation of cutin and wax composition in Arabidopsis thaliana.Plant Cell Physiol 55, 455-466. |
| [75] | Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L (1999). CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme.Plant Cell 11, 825-838. |
| [76] | Millar AA, Kunst L (1997). Very-long-chain fatty acid bio- synthesis is controlled through the expression and speci- ficity of the condensing enzyme.Plant J 12, 121-131. |
| [77] | Molina I, Kosma D (2015). Role of HXXXD-motif/BAHD acyltransferases in the biosynthesis of extracellular lipids.Plant Cell Rep 34, 587-601. |
| [78] | Molina I, Ohlrogge JB, Pollard M (2008). Deposition and localization of lipid polyester in developing seeds of Bras- sica napus and Arabidopsis thaliana.Plant J 53, 437-449. |
| [79] | Nawrath C (2002). The biopolymers cutin and suberin.Arabidopsis Book 1, e0021. |
| [80] | Nawrath C (2006). Unraveling the complex network of cuti- cular structure and function.Curr Opin Plant Biol 9, 281-287. |
| [81] | Nawrath C, Schreiber L, Franke RB, Geldner N, Reina- Pinto JJ, Kunst L (2013). Apoplastic diffusion barriers in Arabidopsis.Arabidopsis Book 11, e0167. |
| [82] | Olson DA, Sheares VV (2006). Preparation of unsaturated linear aliphatic polyesters using condensation polymeri- zation.Macromolecules 39, 2808-2814. |
| [83] | Olsson A, Lindström M, Iversen T (2007). Lipase-catalyzed synthesis of an epoxy-functionalized polyester from the suberin monomer cis-9,10-epoxy-18-hydroxyoctadecanoic acid.Biomacromolecules 8, 757-760. |
| [84] | Oshima Y, Shikata M, Koyama T, Ohtsubo N, Mitsuda N, Ohme-Takagi M (2013). MIXTA-like transcription factors and WAX INDUCER1/SHINE1 coordinately regulate cuti- cle development in Arabidopsis and Torenia fournieri.Pl- ant Cell 25, 1609-1624. |
| [85] | Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, Höfer R, Schreiber L, Chory J, Aharoni A (2007). The Arabidopsis DESPERADO/AtWBC11 trans- porter is required for cutin and wax secretion.Plant Physiol 145, 1345-1360. |
| [86] | Panikashvili D, Shi JX, Bocobza S, Franke RB, Schreiber L, Aharoni A (2010). The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots.Mol Plant 3, 563-575. |
| [87] | Panikashvili D, Shi JX, Schreiber L, Aharoni A (2009). The Arabidopsis DCR encoding a soluble BAHD acyltransfe- rase is required for cutin polyester formation and seed hy- dration properties.Plant Physiol 151, 1773-1789. |
| [88] | Panikashvili D, Shi JX, Schreiber L, Aharoni A (2011). The Arabidopsis ABCG13 transporter is required for flower cuticle secretion and patterning of the petal epidermis.New Phytol 190, 113-124. |
| [89] | Pighin JA, Zheng HQ, Balakshin LJ, Goodman IP, Wes- tern TL, Jetter R, Kunst L, Samuels AL (2004). Plant cuticular lipid export requires an ABC transporter.Science 306, 702-704. |
| [90] | Pollard M, Beisson F, Li YH, Ohlrogge JB (2008). Building lipid barriers: biosynthesis of cutin and suberin.Trends Plant Sci 13, 236-246. |
| [91] | Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ (2000). FIDDLEHEAD, a gene required to sup- press epidermal cell interactions in Arabidopsis, en- codes a putative lipid biosynthetic enzyme.Proc Natl Acad Sci USA 97, 1311-1316. |
| [92] | Pulsifer IP, Kluge S, Rowland O (2012). Arabidopsis long- chain acyl-CoA synthetase 1 (LACS1), LACS2, and LA- CS3 facilitate fatty acid uptake in yeast.Plant Physiol Bio- chem 51, 31-39. |
| [93] | Quist TM, Sokolchik I, Shi HZ, Joly RJ, Bressan RA, Maggio A, Narsimhan M, Li X (2009). HOS3, an ELO-like gene, inhibits effects of ABA and implicates a S-1-P/ ceramide control system for abiotic stress responses in Arabidopsis thaliana.Mol Plant 2, 138-151. |
| [94] | Raffaele S, Vailleau F, Léger A, Joubès J, Miersch O, Huard C, Blée E, Mongrand S, Domergue F, Roby D (2008). A MYB transcription factor regulates very-long- chain fatty acid biosynthesis for activation of the hyper- sensitive cell death response in Arabidopsis.Plant Cell 20, 752-767. |
| [95] | Rani SH, Krishna THA, Saha S, Negi AS, Rajasekharan R (2010). Defective in cuticular ridges (DCR) of Arabidopsis thaliana, a gene associated with surface cutin formation, encodes a soluble diacylglycerol acyltransferase.J Biol Chem 285, 38337-38347. |
| [96] | Rautengarten C, Ebert B, Ouellet M, Nafisi M, Baidoo EEK, Benke P, Stranne M, Mukhopadhyay A, Keasling JD, Sakuragi Y, Scheller HV (2012). Arabidopsis deficient in cutinferulate encodes a transferase required for feru- loylation of ω-hydroxy fatty acids in cutin polyester.Plant Physiol 158, 654-665. |
| [97] | Rowland O, Lee R, Franke R, Schreiber L, Kunst L (2007). The CER3 wax biosynthetic gene from Arabidopsis tha- liana is allelic to WAX2/YRE/FLP1.FEBS Lett 581, 3538-3544. |
| [98] | Rowland O, Zheng HQ, Hepworth SR, Lam P, Jetter R, Kunst L (2006). CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax pro- duction in Arabidopsis.Plant Physiol 142, 866-877. |
| [99] | Schnurr J, Shockey J, Browse J (2004). The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis.Plant Cell 16, 629-642. |
| [100] | Seo PJ, Lee SB, Suh MC, Park MJ, Go YS, Park CM (2011). The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis.Plant Cell 23, 1138-1152. |
| [101] | Serrano M, Coluccia F, Torres M, L’Haridon F, Métraux J (2014). The cuticle and plant defense to pathogens.Front Plant Sci 5, 274. |
| [102] | Shi JX, Malitsky S, De Oliveira S, Branigan C, Franke RB, Schreiber L, Aharoni A (2011). SHINE transcription factors act redundantly to pattern the archetypal surface of Arabidopsis flower organs.PLoS Genet 7, e1001388. |
| [103] | Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Métraux JP, Nawrath C (2000). Transgenic Arabidop- sis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions.Plant Cell 12, 721-737. |
| [104] | Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F (2005). Cuticular lipid composi- tion, surface structure, and gene expression in Arabidopsis stem epidermis.Plant Physiol 139, 1649-1665. |
| [105] | Todd J, Post-Beittenmiller D, Jaworski JG (1999). KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana.Plant J 17, 119-130. |
| [106] | Van den Brûle S, Smart CC (2002). The plant PDR family of ABC transporters.Planta 216, 95-106. |
| [107] | Voisin D, Nawrath C, Kurdyukov S, Franke RB, Reina- Pinto JJ, Efremova N, Will I, Schreiber L, Yephremov A (2009). Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator.PLoS Genet 5, e1000703. |
| [108] | Weng H, Molina I, Shockey J, John B (2010). Organ fusion and defective cuticle function in a lacs1 lacs2 double mutant of Arabidopsis.Planta 231, 1089-1100. |
| [109] | Wu RH, Li SB, He S, Wassmann F, Yu CH, Qin GJ, Schreiber L, Qu LJ, Gu HY (2011). CFL1, a WW domain protein, regulates cuticle development by modulating the function of HDG1, a class IV homeodomain transcription factor, in rice and Arabidopsis.Plant Cell 23, 3392-3411. |
| [110] | Xia Y, Yu KS, Navarre D, Seebold K, Kachroo A, Kachroo P (2010). The glabra1 mutation affects cuticle formation and plant responses to microbes.Plant Physiol 154, 833-846. |
| [111] | Xiao FM, Goodwin SM, Xiao YM, Sun ZY, Baker D, Tang XY, Jenks MA, Zhou JM (2004). Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development.EMBO J 23, 2903-2913. |
| [112] | Xue Y, Xiao S, Kim J, Lung SC, Chen L, Tanner JA, Suh MC, Chye ML (2014). Arabidopsis membrane-associated acyl-CoA-binding protein ACBP1 is involved in stem cuticle formation.J Exp Bot 65, 5473-5483. |
| [113] | Yang WL, Simpson JP, Li-Beisson Y, Beisson F, Pollard M, Ohlrogge JB (2012). A land-plant-specific glycerol-3- phosphate acyltransferase family in Arabidopsis: substrate specificity, sn-2 preference, and evolution.Plant Physiol 160, 638-652. |
| [114] | Yang ZJ, Zhang T, Lang T, Li GR, Chen GX, Nevo E (2013). Transcriptome comparative profiling of barley eibi1 mutant reveals pleiotropic effects of HvABCG31 gene on cuticle biogenesis and stress responsive pathways.Inter J Mol Sci 14, 20478-20491. |
| [115] | Yeats TH, Buda GJ, Wang ZH, Chehanovsky N, Moyle LC, Jetter R, Schaffer AA, Rose JKC (2012a). The fruit cuticles of wild tomato species exhibit architectural and chemical diversity, providing a new model for studying the evolution of cuticle function.Plant J 69, 655-666. |
| [116] | Yeats TH, Howe KJ, Matas AJ, Buda GJ, Thannhauser TW, Rose JKC (2010). Mining the surface proteome of tomato (Solanum lycopersicum) fruit for proteins associa- ted with cuticle biogenesis.J Exp Bot 61, 3759-3771. |
| [117] | Yeats TH, Huang WL, Chatterjee S, Viart HMF, Clausen MH, Stark RE, Rose JKC (2014). Tomato Cutin Deficient 1 (CD1) and putative orthologs comprise an ancient family of cutin synthase-like (CUS) proteins that are conserved among land plants.Plant J 77, 667-675. |
| [118] | Yeats TH, Martin LBB, Viart HMF, Isaacson T, He YH, Zhao LX, Matas AJ, Buda GJ, Domozych DS, Clausen MH, Rose JKC (2012b). The identification of cutin synth- ase: formation of the plant polyester cutin.Nat Chem Biol 8, 609-611. |
| [119] | Yeats TH, Rose JKC (2013). The formation and function of plant cuticles.Plant Physiol 163, 5-20. |
| [120] | Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H (1999). Characterization of the FIDDLEH- EAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis.Plant Cell 11, 2187-2201. |
| [121] | Zheng HQ, Rowland O, Kunst L (2005). Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expan- sion during plant morphogenesis.Plant Cell 17, 1467-1481. |
/
| 〈 |
|
〉 |