

Molecular Evolution and Expression Analysis of the OsMIP1 Response to Abiotic Stress
# Co-first authors
Received date: 2016-04-16
Accepted date: 2016-05-26
Online published: 2017-01-23
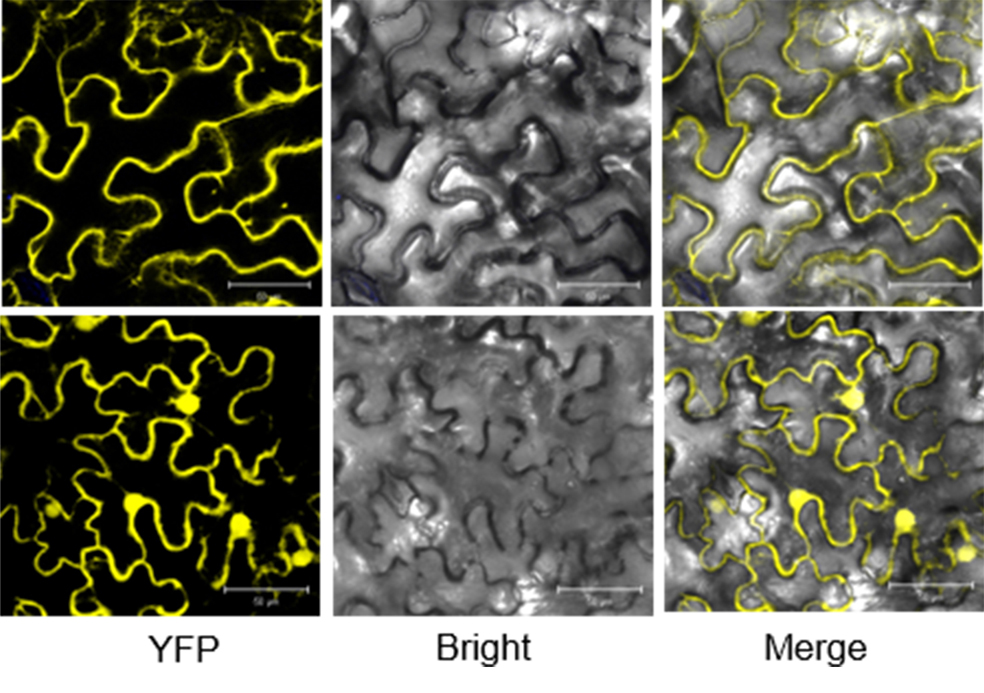
The gene MID1 (MYB IMPORTANT FOR DROUGHT RESPONSE1) encodes a putative R-R type MYB transcription factor; is induced by abiotic stresses, especially drought in reproductive stage; and can improve pollen fertility and rice production. To understand the role of MID1 in abiotic stress responses, we used the yeast two-hybrid system to find an interacting protein, OsMIP1 (Oryza sativa MID1 interaction protein 1). The interaction was further confirmed by BiFC (bimolecular fluorescence complementation) analysis in tobacco leaf cells. OsMIP1 encodes a putative transmembrane protein with an ENTH/ANTH/VHS domain. It is expressed in the root, stem, leaf, panicle and endosperm. Under drought stress, its expression is upregulated in leaf and reproductive organs, especially in post-meiotic flowers. OsMIP1 may play a role in response to drought stress during reproductive development. OsMIP1 expression during vegetative development can be induced by other abiotic stress, including NaCl and mannitol, which suggests that OsMIP1 can respond to other abiotic stresses. There is little analysis of the evolution of genes encoding proteins with the ENTH/ ANTH/VHS domain, so we analyzed the molecular evolution of MIP1 homologs in flowering plants. The evolution analysis of the MIP1 family in angiosperms showed that MIP1 homologs can be divided into 6 types, which originated from at least 6 copies of MIP1 homologous genes in the ancestor of extent angiosperms. After gene-duplication and -loss events, MIP1 family members widely distributed in the angiosperms and might have various functions, possibly in stress responses.
Wang Ling, Guo Changkui, Ren Ding, Ma Hong . Molecular Evolution and Expression Analysis of the OsMIP1 Response to Abiotic Stress[J]. Chinese Bulletin of Botany, 2017 , 52(1) : 43 -53 . DOI: 10.11983/CBB16081
| [1] | Baisa GA, Mayers JR, Bednarek SY (2013). Budding and braking news about clathrin-mediated endocytosis.Curr Opin Plant Biol 16, 718-725. |
| [2] | Barnabas B, Jager K, Feher A (2008). The effect of drought and heat stress on reproductive processes in cereals.Plant Cell Environ 31, 11-38. |
| [3] | Cantrell RP, Reeves TG (2002). The rice genome. The cereal of the world's poor takes center stage.Science 296, 53. |
| [4] | Chen YH, Yang XY, He K, Liu MH, Li JG, Gao ZF, Lin ZQ, Zhang YF, Wang XX, Qiu XX, Shen YP, Zhang Li, Deng XH, Luo JC, Deng XW, Chen ZL, Gu HY, Qu LJ (2006). The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family.Plant Mol Biol 60, 107-124. |
| [5] | Fang YJ, Xie KB, Hou X, Hu HH, Xiong LH (2010). Systematic analysis of GT factor family of rice reveals a novel subfamily involved in stress responses.Mol Genet Genomics 283, 157-169. |
| [6] | Guo CK, You CJ, Wang SS, Cui J, Ge XC, Ma H (2016). MID1 plays an important role in response to drought stress during reproductive development. Plant J 88, 280-293. |
| [7] | Hadiarto T, Tran LS (2011). Progress studies of drought- responsive genes in rice. Plant Cell Rep 30, 297-310. |
| [8] | Jin Y, Yang H, Wei Z, Ma H, Ge X (2013). Rice male deve- lopment under drought stress: phenotypic changes and stage-dependent transcriptomic reprogramming.Mol Plant 6, 1630-1645. |
| [9] | Lee HK, Cho SK, Son O, Xu ZY, Hwang I, Kim WT (2009). Drought stress-induced Rma1H1, a RING membrane- anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants.Plant Cell 21, 622-641. |
| [10] | Li H, Yuan Z, Vizcay-Barrena G, Yang C, Liang W, Zong J, Wilson ZA, Zhang DB (2011). PERSISTENT TAPET- AL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice.Plant Physiol 156, 615-630. |
| [11] | Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, Gwadz M, Hao L, He S, Hurwitz DI, Jackson JD, Ke Z, Krylov D, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Thanki N, Yamashita RA, Yin JJ, Zhang D, Bryant SH (2007). CDD: a conserved domain database for interactive domain family analysis.Nucleic Acids Res 35, D237-D240. |
| [12] | Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003). Regulatory network of gene expression in the drought and cold stress responses.Curr Opin Plant Biol 6, 410-417. |
| [13] | Su Z, Ma X, Guo H, Sukiran NL, Guo B, Assmann SM, Ma H (2013). Flower development under drought stress: morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis.Plant Cell 25, 3785-3807. |
| [14] | Wang D, Pan Y, Zhao X, Zhu L, Fu B, Li Z (2011). Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice.BMC Genomics 12, 149. |
| [15] | Xiong J, Zhang L, Fu GF, Yang YJ, Zhu C, Tao L (2012). Drought-induced proline accumulation is uninvolved with increased nitric oxide, which alleviates drought stress by decreasing transpiration in rice.J Plant Res 125, 155-164. |
| [16] | Zeng L, Zhang Q, Sun R, Kong H, Zhang N, Ma H (2014). Resolving deep angiosperm phylogenetic relationships using conserved nuclear genes.Nat Commun 5, 4956. |
| [17] | Zhu QH, Ramm K, Shivakkumar R, Dennis ES, Upad- hyaya NM (2004). The ANTHER INDEHISCENCE1 gene encoding a single MYB domain protein is involved in anther development in rice.Plant Physiol 135, 1514-1525. |
| [18] | Zouhar J, Sauer M (2014). Helping hands for budding prospects: ENTH/ANTH/VHS accessory proteins in endocytosis, vacuolar transport, and secretion.Plant Cell 26, 4232-4244. |
/
| 〈 |
|
〉 |