

Optimization of an High-performance Liquid Chromatography Method for the Determination of Salicylic Acid
Received date: 2025-06-04
Accepted date: 2025-07-08
Online published: 2025-07-08
INTRODUCTION: The phytohormone salicylic acid (SA) plays multiple important roles in plants, such as disease resistance, seed germination, and leaf senescence. Among these, the role of SA in plant disease resistance is the most studied. Since SA promotes disease resistance at the cost of plant growth, plants need to dynamically regulate the content of SA to balance disease resistance and growth. Therefore, fast and accurate measurement of SA content is a critical basis for plant immunity research.
RATIONALE: High-performance liquid chromatography (HPLC)-fluorescence detector is the most popular method for the quantitative measurement of SA. In order to improve the efficiency and sensitivity of current methods, this study optimized the composition, ion concentration, and pH of the mobile phase, as well as the detection wavelength and detection procedure.
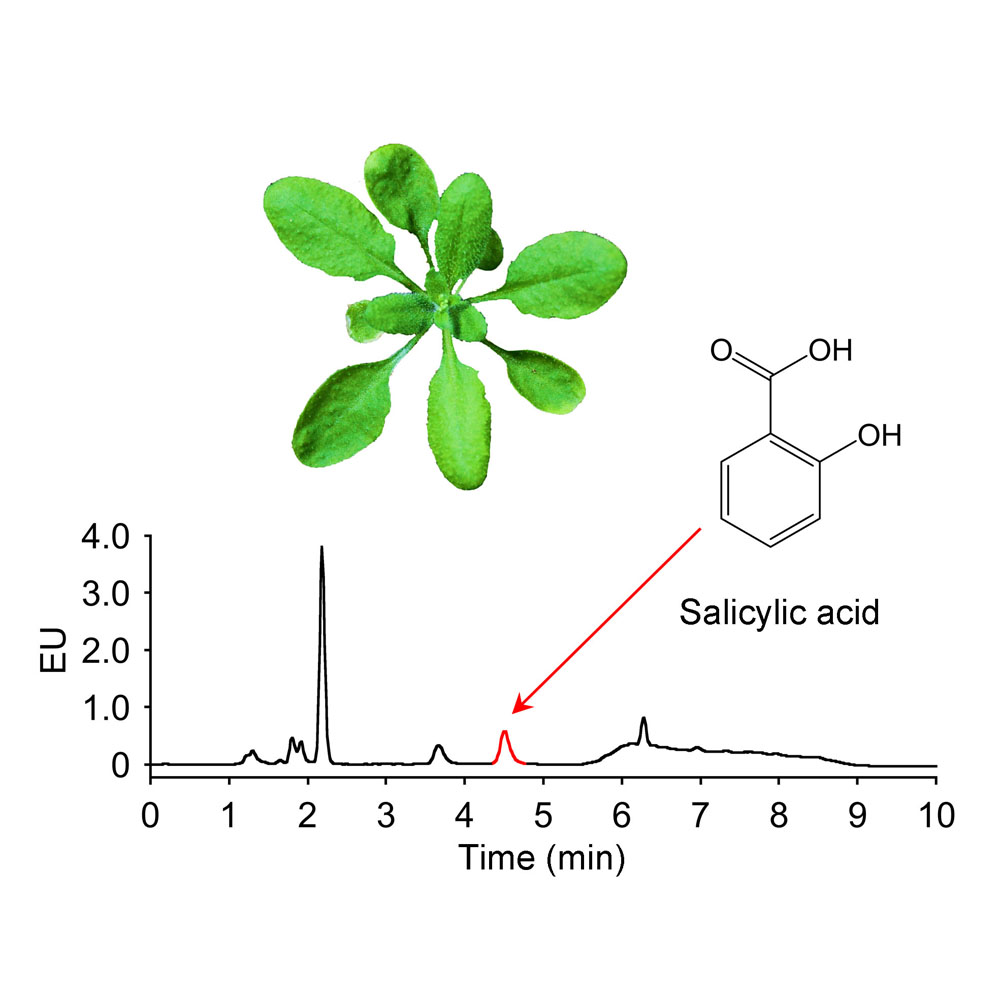
RESULTS: The baseline of the chromatogram was more stable when using acetonitrile instead of methanol in the mobile phase. When the pH of the mobile phase was 5.2, the retention time of SA was the shortest, without interference peak near the SA peak, which was preferred for minimizing the detection time. The higher concentration of sodium acetate (100 mmol∙L-1) in the mobile phase was better than that of lower concentration (20-50 mmol∙L-1). Wavelength scanning revealed that the optimal excitation wavelength was 300 nm and the optimal emission wavelength was 405 nm, under which the highest sensitivity for SA detection was obtained. At a flow rate of 2 mL∙min-1, it took 3.5 min for elution, 3.5 min for column wash, and 3 min for column balance, shortening the measurement time per sample from 50 min to 10 min.
CONCLUSION: These optimizations greatly improved the sensitivity, stability, and efficiency of the SA measurement using HPLC, which will contribute to the plant immunity research.
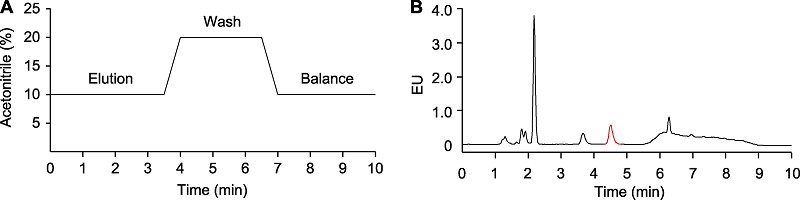
Optimization of salicylic acid (SA) measurement. (A) SA detection procedure; (B) Chromatogram using the optimized condition. The samples are total SA in Arabidopsis without Psm ES4326 infection. EU: Emission units. The peak labeled in red indicates SA.
Shi Shixi , Yan Shunping . Optimization of an High-performance Liquid Chromatography Method for the Determination of Salicylic Acid[J]. Chinese Bulletin of Botany, 2025 , 60(5) : 846 -853 . DOI: 10.11983/CBB25102
| [1] | Aboul-Soud MAM, Cook K, Loake GJ (2004). Measurement of salicylic acid by a high-performance liquid chromatography procedure based on ion-exchange. Chromatographia 59, 129-133. |
| [2] | Balcke GU, Handrick V, Bergau N, Fichtner M, Henning A, Stellmach H, Tissier A, Hause B, Frolov A (2012). An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 8, 47. |
| [3] | Cao H, Bowling SA, Gordon AS, Dong XN (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583-1592. |
| [4] | Defraia CT, Schmelz EA, Mou ZL (2008). A rapid biosensor-based method for quantification of free and glucose- conjugated salicylic acid. Plant Methods 4, 28. |
| [5] | Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J (1994). A central role of salicylic acid in plant disease resistance. Science 266, 1247-1250. |
| [6] | Ding YL, Sun TJ, Ao K, Peng YJ, Zhang YX, Li X, Zhang YL (2018). Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454-1467. |
| [7] | Fu ZQ, Dong XN (2013). Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64, 839-863. |
| [8] | Fu ZQ, Yan SP, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong XN (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228-232. |
| [9] | Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754-756. |
| [10] | Huang WE, Wang H, Zheng HJ, Huang LF, Singer AC, Thompson I, Whiteley AS (2005). Chromosomally located gene fusions constructed in Acinetobacter sp. ADP1 for the detection of salicylate. Environ Microbiol 7, 1339-1348. |
| [11] | Kumar S, Zavaliev R, Wu QL, Zhou Y, Cheng J, Dillard L, Powers J, Withers J, Zhao JS, Guan ZQ, Borgnia MJ, Bartesaghi A, Dong XN, Zhou P (2022). Structural basis of NPR1 in activating plant immunity. Nature 605, 561-566. |
| [12] | Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2002). The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem 277, 586-592. |
| [13] | Marek G, Carver R, Ding YZ, Sathyanarayan D, Zhang XD, Mou ZZ (2010). A high-throughput method for isolation of salicylic acid metabolic mutants. Plant Methods 6, 21. |
| [14] | Mishra S, Roychowdhury R, Ray S, Hada A, Kumar A, Sarker U, Aftab T, Das R (2024). Salicylic acid (SA)-mediated plant immunity against biotic stresses: an insight on molecular components and signaling mechanism. Plant Stress 11, 100427. |
| [15] | Nawrath C, Métraux JP (1999). Salicylic acid induction- deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11, 1393-1404. |
| [16] | Raskin I, Turner IM, Melander WR (1989). Regulation of heat production in the inflorescences of an Arum lily by endogenous salicylic acid. Proc Natl Acad Sci USA 86, 2214-2218. |
| [17] | Schmelz EA, Engelberth J, Alborn HT, O’Donnell P, Sammons M, Toshima H, Tumlinson JH (2003). Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc Natl Acad Sci USA 100, 10552-10557. |
| [18] | Snyder LR, Kirkland JJ, Dolan JW (陈小明, 唐雅妍译) (2012). 现代液相色谱技术导论(第3版). 北京: 人民卫生出版社. pp. 126-131. |
| [19] | Song JT (2006). Induction of a salicylic acid glucosyltransferase, AtSGT1, is an early disease response in Arabidopsis thaliana. Mol Cells 22, 233-238. |
| [20] | Yan SP, Dong XN (2014). Perception of the plant immune signal salicylic acid. Curr Opin Plant Biol 20, 64-68. |
| [21] | Ye C, Yao LB, Jin Y, Gao R, Tan Q, Li XY, Zhang YJ, Chen XF, Ma BJ, Zhang W, Zhang KW (2025). Establishment and application of a high-throughput screening method for salicylic acid metabolic mutants in rice. Chin Bull Bot 60, 586-596. (in Chinese) |
| 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟 (2025). 水稻水杨酸代谢突变体高通量筛选方法的建立与应用. 植物学报 60, 586-596. | |
| [22] | Yu XD, Cui XY, Wu C, Shi SX, Yan SP (2022). Salicylic acid inhibits gibberellin signaling through receptor interactions. Mol Plant 15, 1759-1771. |
| [23] | Yu XD, Xu YR, Yan SP (2021). Salicylic acid and ethylene coordinately promote leaf senescence. J Integr Plant Biol 63, 823-827. |
| [24] | Zhang YJ, Zhao L, Zhao JZ, Li YJ, Wang JB, Guo R, Gan SS, Liu CJ, Zhang KW (2017). S5H/DMR6 encodes a salicylic acid 5-hydroxylase that fine-tunes salicylic acid homeostasis. Plant Physiol 175, 1082-1093. |
/
| 〈 |
|
〉 |