

Expression Pattern and Metabolic Correlation Analysis of TCP Gene Family in Bergenia purpurascens
1College of Life Science and Engineering, Southwest Jiaotong University, Chengdu 610031, China; 2Chengdu Shishi High School of Sichuan Province, Chengdu 610041, China
Received date: 2025-01-16
Revised date: 2025-04-22
Online published: 2025-05-14
INTRODUCTION: The TCP
protein family is a plant-specific group of transcription factors known to
regulate key biological processes, including growth, development, and stress
responses. Despite their critical roles, the TCP gene family in Bergenia
purpurascens remains uncharacterized. This study aims to systematically
identify and analyze the BpTCP gene family in B. purpurascens using
transcriptome-based bioinformatics approaches, providing insights into their
potential functions in cold adaptation and secondary metabolism.
RATIONALE: B. purpurascens exhibits remarkable resilience to abiotic stresses, particularly cold, and contains abundant secondary metabolites. Given the documented roles of TCP genes in stress responses and metabolic regulation in other plants, we hypothesized that BpTCP genes may contribute to these traits. A comprehensive analysis of this gene family could reveal novel mechanisms underlying stress adaptation and metabolite synthesis, supporting future genetic improvement or biotechnological applications.
RESULTS: Through transcriptome-based bioinformatics analysis, we identified 16 BpTCP genes in B. purpurascens, which were phylogenetically classified into two major groups, with all members containing conserved TCP domains and closely related proteins sharing similar motif patterns. Tissue-specific expression profiling revealed distinct spatial expression patterns across different tissues, suggesting functional diversification among family members. Notably, partial genes, including BpTCP10, BpTCP1 and BpTCP12, exhibited significant expression changes under cold stress, implying their potential cold-responsive roles. Furthermore, expression levels of specific BpTCP genes correlated significantly with accumulation of various secondary metabolites, particularly flavonoids and phenolics, suggesting their regulatory involvement in metabolic pathways.
CONCLUSION: This study provides the first genome-wide characterization of the BpTCP gene family in B. purpurascens, demonstrating its potential roles in growth, cold stress response, and secondary metabolism. The differential expression of BpTCP genes under stress and their correlation with metabolite levels lay a foundation for future functional studies.
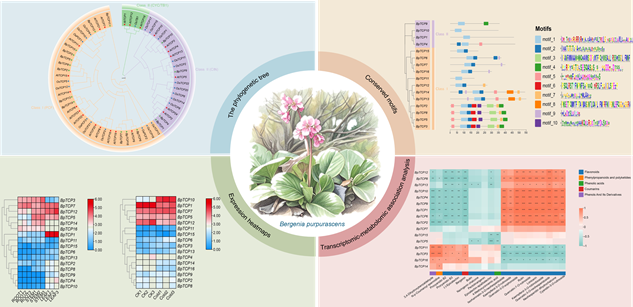
Expression pattern and metabolic correlation analysis of TCP gene family in Bergenia purpurascens. A total of 16 BpTCP genes were identified in Bergenia purpurascens and classified into two major phylogenetic groups. All BpTCP genes contain conserved TCP domains, and proteins from the same evolutionary branch share similar motif compositions. Different BpTCP genes exhibit distinct tissue-specific expression patterns and display distinctive responses to cold stress. Furthermore, certain BpTCP genes demonstrate significant correlations with the accumulation of diverse metabolites.
CHEN Jing-Yu , YU Wen-Qiang , LUO Shi-Yu , YANG Lu-Xiang , WANG Hui-Jun , WU Tian-Yu , ZHU Qian-Kun . Expression Pattern and Metabolic Correlation Analysis of TCP Gene Family in Bergenia purpurascens[J]. Chinese Bulletin of Botany, 0 : 1 -0 . DOI: 10.11983/CBB25008
/
| 〈 |
|
〉 |