

高温胁迫对植物光合作用的影响研究进展
收稿日期: 2022-04-20
录用日期: 2022-07-06
网络出版日期: 2022-07-27
基金资助
国家重点研发计划政府间国际科技创新合作专项(2019YFE0116500)
Research Advances on the Effect of High Temperature Stress on Plant Photosynthesis
Received date: 2022-04-20
Accepted date: 2022-07-06
Online published: 2022-07-27
随着人为活动产生的大气CO2浓度的增加, 全球气候持续变暖。过去5年是自有温度记录以来最热的5年。高温胁迫已经成为影响植物生长发育的主要逆境因子之一。光合作用是地球生命活动的基础, 对环境波动高度敏感。解析植物在高温环境下光合作用的响应特性, 可为探索植物抵御高温的生理生态机制、培育抗高温新品种以及采取合理措施适应未来极端气候提供科学依据。该文论述了高温胁迫对植物光合电子传递及碳固定过程的影响, 从光质和光强角度综合分析了光照对高温胁迫下光合作用的影响; 从植物自身及外源缓解物质等方面阐述了植物增强抗高温胁迫的途径和机制。同时, 对植物光合作用响应高温胁迫的研究方向及多组学联合分析在揭示植物抵御高温胁迫机制中的应用进行了展望。
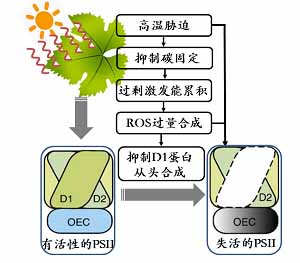
孙永江 , 王琪 , 邵琪雯 , 辛智鸣 , 肖辉杰 , 程瑾 . 高温胁迫对植物光合作用的影响研究进展[J]. 植物学报, 2023 , 58(3) : 486 -498 . DOI: 10.11983/CBB22079
With the increase in atmospheric CO2 concentration caused by human activities, the global climate continues to warm. The past five years have been the hottest since the record of temperature. High temperature stress has become one of the main adverse factors affecting plant growth and development. Photosynthesis is the basis of life activities on earth, and it is highly sensitive to fluctuation in environmental factors. Understanding the response of plant photosynthesis under high temperature stress can provide a scientific basis for exploring the physiological and ecological mechanisms of plant tolerance to high temperature stress, cultivating new heat-tolerant varieties and taking reasonable measures to adapt to extreme climate in the future. In this paper, the effects of high temperature stress on the process of photosynthetic electron transfer and carbon fixation in plants were reviewed, and the effects of light on photosynthesis under high temperature stress were comprehensively analyzed from the perspective of light quality and light intensity. This paper also expounded the ways and mechanisms to improve the tolerance of plants to high temperature stress from the aspects of plants themselves and exogenous mitigating substances. Meanwhile, the research direction of plant photosynthesis response to high temperature stress and the application of multi-histology combined analysis in the comprehensive study of the mechanism of plant tolerance to high temperature stress were prospected.

| [1] | 毕焕改, 李福德, 董绪兵, 艾希珍 (2017). 转酮醇酶基因沉默对高温胁迫下黄瓜幼苗光合作用的影响. 植物生理学报 53, 1859-1866. |
| [2] | 程超华, 唐蜻, 邓灿辉, 戴志刚, 许英, 杨泽茂, 刘婵, 吴姗, 粟建光 (2020). 表型组学及多组学联合分析在植物种质资源精准鉴定中的应用. 分子植物育种 18, 2747-2753. |
| [3] | 黄伟, 张石宝, 曹坤芳 (2012). 高等植物环式电子传递的生理作用. 植物科学学报 30, 100-106. |
| [4] | 姜振升, 孙晓琦, 艾希珍, 王美玲, 毕焕改, 王洪涛 (2010). 低温弱光对黄瓜幼苗Rubisco与Rubisco活化酶的影响. 应用生态学报 21, 2045-2050. |
| [5] | 刘玉凤, 鹿嘉智, 孟思达, 王珍琪, 张耀丰, 王峰, 齐明芳, 李天来 (2019). PGR5/PGRL1介导的环式电子传递研究进展. 植物生理学报 55, 433-443. |
| [6] | 王浩, 王明, 梁婷, 姚玉新, 杜远鹏, 高振 (2022). 气温和根区温度对葡萄叶片光合荧光特性的影响. 植物学报 57, 209-216. |
| [7] | 许大全 (2002). 光合作用效率. 上海: 上海科学技术出版社. pp. 4-5. |
| [8] | Aihara Y, Takahashi S, Minagawa J (2016). Heat induction of cyclic electron flow around photosystem I in the symbio- tic dinoflagellate Symbiodinium. Plant Physiol 171, 522-529. |
| [9] | Alayafi AAM (2020). Exogenous ascorbic acid induces systemic heat stress tolerance in tomato seedlings: transcriptional regulation mechanism. Environ Sci Pollut Res Int 27, 19186-19199. |
| [10] | Allen JF (1995). Thylakoid protein phosphorylation, state 1- state 2 transitions, and photosystem stoichiometry adjustment: redox control at multiple levels of gene expression. Physiol Plant 93, 196-205. |
| [11] | Arico D, Legris M, Castro L, Garcia CF, Laino A, Casal JJ, Mazzella MA (2019). Neighbour signals perceived by phytochrome B increase thermotolerance in Arabidopsis. Plant Cell Environ 42, 2554-2566. |
| [12] | Atkin OK, Bruhn D, Hurry VM, Tjoelker MG (2005). The hot and the cold: unravelling the variable response of plant respiration to temperature. Funct Plant Biol 32, 87-105. |
| [13] | Berry J, Bjorkman O (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31, 491-543. |
| [14] | Bracher A, Whitney SM, Hartl FU, Hayer-Hartl M (2017). Biogenesis and metabolic maintenance of Rubisco. Annu Rev Plant Biol 68, 29-60. |
| [15] | Buchner O, Stoll M, Karadar M, Kranner I, Neuner G (2015). Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic perfor-mance in alpine plants. Plant Cell Environ 38, 812-826. |
| [16] | Cao YY, Zhao H (2008). Protective roles of brassinolide on rice seedlings under high temperature stress. Rice Sci 15, 63-68. |
| [17] | Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT (2007). A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143, 251-262. |
| [18] | Chen JH, Chen ST, He NY, Wang QL, Zhao Y, Gao W, Guo FQ (2020). Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat Plants 6, 570-580. |
| [19] | Chen JH, Tang M, Jin XQ, Li H, Chen LS, Wang QL, Sun AZ, Yi Y, Guo FQ (2022). Regulation of Calvin-Benson cycle enzymes under high temperature stress. aBIOTECH 3, 65-77. |
| [20] | Chen ST, He NY, Chen JH, Guo FQ (2017). Identification of core subunits of photosystem II as action sites of HSP21, which is activated by the GUN5-mediated retrograde path- way in Arabidopsis. Plant J 89, 1106-1118. |
| [21] | Chinthapalli B, Murmu J, Raghavendra AS (2003). Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. J Exp Bot 54, 707-714. |
| [22] | Crafts-Brandner SJ, Salvucci ME (2000). Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97, 13430-13435. |
| [23] | Demirevska-Kepova K, Holzer R, Simova-Stoilova L, Feller U (2005). Heat stress effects on ribulose-1,5- bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol Plant 49, 521-525. |
| [24] | DeRidder BP, Salvucci ME (2007). Modulation of Rubisco activase gene expression during heat stress in cotton (Gossypium hirsutum L.) involves post-transcriptional mechanisms. Plant Sci 172, 246-254. |
| [25] | Essemine J, Qu MN, Mi HL, Zhu XG (2016). Response of chloroplast NAD(P)H dehydrogenase-mediated cyclic elec- tron flow to a shortage or lack in ferredoxin-quinone oxidoreductase-dependent pathway in rice following short- term heat stress. Front Plant Sci 7, 383. |
| [26] | Farquhar GD, Von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78-90. |
| [27] | Fauset S, Freitas HC, Galbraith DR, Sullivan MJP, Aidar MPM, Joly CA, Phillips OL, Vieira SA, Gloor MU (2018). Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ 41, 1618-1631. |
| [28] | Feng LL, Wang K, Li Y, Tan YP, Kong J, Li H, Li YS, Zhu YG (2007). Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep 26, 1635-1646. |
| [29] | Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012). Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63, 1637-1661. |
| [30] | Gounaris K, Brain ARR, Quinn PJ, Williams WP (1984). Structural reorganisation of chloroplast thylakoid membranes in response to heat-stress. Biochim Biophys Acta Bioenerg 766, 198-208. |
| [31] | Grabsztunowicz M, Koskela MM, Mulo P (2017). Post- translational modifications in regulation of chloroplast function: recent advances. Front Plant Sci 8, 240. |
| [32] | Haldimann P, Feller U (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) lea-ves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxy- genase. Plant Cell Environ 27, 1169-1183. |
| [33] | Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013). Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14, 9643-9684. |
| [34] | Hashimoto K, Kudla J (2011). Calcium decoding mechanisms in plants. Biochimie 93, 2054-2059. |
| [35] | Havaux M, Greppin H, Strasser RJ (1991). Functioning of photosystems I and II in pea leaves exposed to heat stress in the presence or absence of light: analysis using in vivo fluorescence, absorbance, oxygen and photoa- coustic measurements. Planta 186, 88-98. |
| [36] | Hill R, Bendall FAY (1960). Function of the two cytochrome components in chloroplasts: a working hypothesis. Nature 186, 136-137. |
| [37] | Hu LX, Zhang ZF, Xiang ZX, Yang ZJ (2016). Exogenous application of citric acid ameliorates the adverse effect of heat stress in tall fescue (Lolium arundinaceum). Front Plant Sci 7, 179. |
| [38] | Hüve K, Bichele I, Rasulov B, Niinemets ü (2011). When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ 34, 113-126. |
| [39] | IPCC (2021). Climate Change 2021: the Physical Science Basis. Cambridge: Cambridge University Press. pp. 41. |
| [40] | Jahan MS, Guo SR, Sun J, Shu S, Wang Y, El-Yazied AA, Alabdallah NM, Hikal M, Mohamed MHM, Ibrahim MFM, Hasan MM (2021). Melatonin-mediated photosynthetic performance of tomato seedlings under high-tem- perature stress. Plant Physiol Biochem 167, 309-320. |
| [41] | Jiang Y, Feng X, Wang H, Chen YQ, Sun YJ (2021). Heat-induced down-regulation of photosystem II protects photosystem I in honeysuckle (Lonicera japonica). J Plant Res 134, 1311-1321. |
| [42] | Joly D, Carpentier R (2007). Regulation of energy dissipation in photosystem I by the redox state of the plasto- quinone pool. Biochemistry 46, 5534-5541. |
| [43] | Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao MJ, Khattak AK, Box MS, Charoensawan V, Cortijo S, Kumar M, Grant A, Locke JCW, Sch?fer E, Jaeger KE, Wigge PA (2016). Phytochromes function as thermosensors in Arabidopsis. Science 354, 886-889. |
| [44] | Kallis RP, Ewy RG, Portis AR Jr (2000). Alteration of the adenine nucleotide response and increased Rubisco activation activity of Arabidopsis Rubisco activase by site- directed mutagenesis. Plant Physiol 123, 1077-1086. |
| [45] | Kao WY, Forseth IN (1992). Dirunal leaf movement, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant Cell Environ 15, 703-710. |
| [46] | Lascano HR, Casano LM, Martin M, Sabater B (2003). The activity of the chloroplastic NDH complex is regulated by phosphorylation of the NDH-F subunit. Plant Physiol 132, 256-262. |
| [47] | Lau OS, Song ZJ, Zhou ZM, Davies KA, Chang J, Yang X, Wang SQ, Lucyshyn D, Tay IHZ, Wigge PA, Bergmann DC (2018). Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr Biol 28, 1273-1280. |
| [48] | Le Quéré C, Jackson RB, Jones MW, Smith AJP, Abernethy S, Andrew RM, De-Gol AJ, Willis DR, Shan YL, Canadell JG, Friedlingstein P, Creutzig F, Peters GP (2020). Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nat Clim Change 10, 647-653. |
| [49] | Lee DG, Ahsan N, Lee SH, Kang KY, Bahk JD, Lee IJ, Lee BH (2007). A proteomic approach in analyzing heat- responsive proteins in rice leaves. Proteomics 7, 3369-3383. |
| [50] | Legris M, Nieto C, Sellaro R, Prat S, Casal JJ (2017). Perception and signaling of light and temperature cues in plants. Plant J 90, 683-697. |
| [51] | Li N, Bo CP, Zhang YY, Wang L (2021). PHYTOCHROME INTERACTING FACTORS PIF4 and PIF5 promote heat stress induced leaf senescence in Arabidopsis. J Exp Bot 72, 4577-4589. |
| [52] | Li PM, Cheng LL, Gao HY, Jiang CD, Peng T (2009). Hete- rogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. J Plant Physiol 166, 1607-1615. |
| [53] | Luo Y, Xie Y, He D, Wang W, Yuan S (2021). Exogenous trehalose protects photosystem II by promoting cyclic electron flow under heat and drought stresses in winter wheat. Plant Biol 23, 770-776. |
| [54] | Marutani Y, Yamauchi Y, Kimura Y, Mizutani M, Sugimoto Y (2012). Damage to photosystem II due to heat stress without light-driven electron flow: involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 236, 753-761. |
| [55] | Mathur S, Allakhverdiev SI, Jajoo A (2011). Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of photosystem II in wheat (Triticum aestivum) leaves. Biochim Biophys Acta Bioenerg 1807, 22-29. |
| [56] | Maxwell DP, Laudenbach DE, Huner N (1995). Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol 109, 787-795. |
| [57] | Mayton H, Myers K, Fry WE (2008). Potato late blight in tubers—the role of foliar phosphonate applications in suppressing pre-harvest tuber infections. Crop Prot 27, 943-950. |
| [58] | Mittler R, Finka A, Goloubinoff P (2012). How do plants feel the heat? Trends Biochem Sci 37, 118-125. |
| [59] | Mohanty S, Verma SK, Nayak SK (2006). Dynamic mechanical and thermal properties of MAPE treated jute/ HDPE composites. Compos Sci Technol 66, 538-547. |
| [60] | Müller P, Li XP, Niyogi KK (2001). Non-photochemical quenching. A response to excess light energy. Plant Phy- siol 125, 1558-1566. |
| [61] | Munekage Y, Hashimoto M, Miyake C, Tomizawa KI, Endo T, Tasaka M, Shikanai T (2004). Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429, 579-582. |
| [62] | Murchie EH, Hubbart S, Peng S, Horton P (2005). Acclimation of photosynthesis to high irradiance in rice: gene expression and interactions with leaf development. J Exp Bot 56, 449-460. |
| [63] | Nishiyama Y, Allakhverdiev SI, Murata N (2006). A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta Bioenerg 1757, 742-749. |
| [64] | Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20, 5587-5594. |
| [65] | Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH, Yu JQ, Nogués S (2008). Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul 27, 49-57. |
| [66] | Osei-Bonsu I, McClain AM, Walker BJ, Sharkey TD, Kramer DM (2021). The roles of photorespiration and alternative electron acceptors in the responses of photosynthesis to elevated temperatures in cowpea. Plant Cell Environ 44, 2290-2307. |
| [67] | Oyarburo NS, Machinandiarena MF, Feldman ML, Daleo GR, Andreu AB, Olivieri FP (2015). Potassium phos- phite increases tolerance to UV-B in potato. Plant Physiol Biochem 88, 1-8. |
| [68] | Perkins-Kirkpatrick SE, Lewis SC (2020). Increasing trends in regional heatwaves. Nat Commun 11, 3357. |
| [69] | Pollastri S, Sukiran NA, Jacobs BCIC, Knight MR (2021). Chloroplast calcium signaling regulates thermomemory. J Plant Physiol 264, 153470. |
| [70] | Pshybytko NL, Kruk J, Kabashnikova LF, Strzalka K (2008). Function of plastoquinone in heat stress reactions of plants. Biochim Biophys Acta Bioenerg 1777, 1393-1399. |
| [71] | Qazi HA, Jan N, Ramazan S, John R (2019). Protein modification in plants in response to abiotic stress. In: Dar TA, Singh LR, eds. Protein Modificomics. Amsterdam: Else- vier. pp. 171-201. |
| [72] | Qu AL, Ding YF, Jiang Q, Zhu C (2013). Molecular mechanisms of the plant heat stress response. Biochem Bioph Res Commun 432, 203-207. |
| [73] | Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, Van Zanten M (2016). Molecular and genetic control of plant thermomorphogenesis. Nat Plants 2, 15190. |
| [74] | Richter K, Haslbeck M, Buchner J (2010). The heat shock response: life on the verge of death. Mol Cell 40, 253-266. |
| [75] | Ruban AV (2016). Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physiol 170, 1903-1916. |
| [76] | Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton GGR, Chow WS, Anderson JM, Osmond CB (1995). Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol 107, 943-952. |
| [77] | Rytz TC, Miller MJ, McLoughlin F, Augustine RC, Marshall RS, Juan YT, Charng YY, Scalf M, Smith LM, Vierstra RD (2018). SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO liga- se SIZ1 during heat stress. Plant Cell 30, 1077-1099. |
| [78] | Sadura I, Libik-Konieczny M, Jurczyk B, Gruszka D, Janeczko A (2020). HSP transcript and protein accumulation in brassinosteroid barley mutants acclimated to low and high temperatures. Int J Mol Sci 21, 1889. |
| [79] | Sajid M, Rashid B, Ali Q, Husnain T (2018). Mechanisms of heat sensing and responses in plants. It is not all about Ca2+ ions. Biol Plant 62, 409-420. |
| [80] | Shekhawat K, Almeida-Trapp M, García-Ramírez GX, Hirt H (2022). Beat the heat: plant- and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci 27, 802-813. |
| [81] | Sherstneva O, Khlopkov A, Gromova E, Yudina L, Vetrova Y, Pecherina A, Kuznetsova D, Krutova E, Sukhov V, Vodeneev V (2022). Analysis of chlorophyll fluorescence parameters as predictors of biomass accumulation and tolerance to heat and drought stress of wheat (Triticum aestivum) plants. Funct Plant Biol 49, 155-169. |
| [82] | Siebers MH, Slattery RA, Yendrek CR, Locke AM, Drag D, Ainsworth EA, Bernacchi CJ, Ort DR (2017). Simulated heat waves during maize reproductive stages alter reproductive growth but have no lasting effect when applied during vegetative stages. Agric Ecosyst Environ 240, 162-170. |
| [83] | Slattery RA, Ort DR (2019). Carbon assimilation in crops at high temperatures. Plant Cell Environ 42, 2750-2758. |
| [84] | Song JY, Liu QJ, Hu BR, Wu WJ (2017). Photoreceptor phyB involved in Arabidopsis temperature perception and heat-tolerance formation. Int J Mol Sci 18, 1194. |
| [85] | Sonoike K (2011). Photoinhibition of photosystem I. Physiol Plant 142, 56-64. |
| [86] | Stotz M, Mueller-Cajar O, Ciniawsky S, Wendler P, Hartl FU, Bracher A, Hayer-Hartl M (2011). Structure of green- type Rubisco activase from tobacco. Nat Struct Mol Biol 18, 1366-1370. |
| [87] | Strand DD, Livingston AK, Satoh-Cruz M, Froehlich JE, Maurino VG, Kramer DM (2015). Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci USA 112, 5539-5544. |
| [88] | Sun YJ, Geng QW, Du YP, Yang XH, Zhai H (2017). Induction of cyclic electron flow around photosystem I du- ring heat stress in grape leaves. Plant Sci 256, 65-71. |
| [89] | Suorsa M, Rossi F, Tadini L, Labs M, Colombo M, Jahns P, Kater MM, Leister D, Finazzi G, Aro EM, Barbato R, Pesaresi P (2016). PGR5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol Plant 9, 271-288. |
| [90] | Suzuki K, Nagasuga K, Okada M (2008). The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49, 433-442. |
| [91] | Takahashi S, Badger MR (2011). Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci 16, 53-60. |
| [92] | Takahashi S, Murata N (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13, 178-182. |
| [93] | Tan SL, Yang YJ, Huang W (2020). Moderate heat stress accelerates photoinhibition of photosystem I under fluct- uating light in tobacco young leaves. Photosynth Res 144, 373-382. |
| [94] | Tan W, Meng QW, Brestic M, Olsovska K, Yang XH (2011). Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol 168, 2063-2071. |
| [95] | Tang S, Zhang HX, Li L, Liu X, Chen L, Chen WZ, Ding YF (2018). Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct Plant Biol 45, 911-921. |
| [96] | Tarvainen L, Wittemann M, Mujawamariya M, Manishim- we A, Zibera E, Ntirugulirwa B, Ract C, Manzi OJL, Andersson MX, Spetea C, Nsabimana D, Wallin G, Uddling J (2022). Handling the heat-photosynthetic thermal stress in tropical trees. New Phytol 233, 236-250. |
| [97] | Teicher HB, Scheller HV (1998). The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH. Plant Physiol 117, 525-532. |
| [98] | Thussagunpanit J, Jutamanee K, Kaveeta L, Chai-Arree W, Pankean P, Homvisasevongsa S, Suksamrarn A (2015). Comparative effects of brassinosteroid and brass- inosteroid mimic on improving photosynthesis, lipid peroxidation, and rice seed set under heat stress. J Plant Growth Regul 34, 320-331. |
| [99] | Tikkanen M, Mekala NR, Aro EM (2014). Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim Biophys Acta Bioenerg 1837, 210-215. |
| [100] | Tollefson J (2021). IPCC climate report:earth is warmer than it's been in 125,000 years. Nature 596, 171-172. |
| [101] | Tserej O, Feeley KJ (2021). Variation in leaf temperatures of tropical and subtropical trees are related to leaf thermoregulatory traits and not geographic distributions. Biotro- pica 53, 868-878. |
| [102] | Tyystj?rvi E (2012). Photoinhibition of photosystem II. Int Rev Cell Mol Biol 300, 243-303. |
| [103] | Tyystj?rvi E, Aro EM (1996). The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93, 2213-2218. |
| [104] | Vierling E (1991). The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42, 579-620. |
| [105] | Von Caemmerer S (2020). Rubisco carboxylase/oxyge- nase: from the enzyme to the globe: a gas exchange perspective. J Plant Physiol 252, 153240. |
| [106] | Wada M, Kagawa T, Sato Y (2003). Chloroplast movement. Annu Rev Plant Biol 54, 455-468. |
| [107] | Wang ML, Zhang XY, Li QH, Chen X, Li XH (2019). Comparative transcriptome analysis to elucidate the enhanced thermotolerance of tea plants (Camellia sinensis) treated with exogenous calcium. Planta 249, 775-786. |
| [108] | Wang P, Duan W, Takabayashi A, Endo T, Shikanai T, Ye JY, Mi HL (2006). Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative da- mage caused by temperature stress. Plant Physiol 141, 465-474. |
| [109] | Wang QL, Chen JH, He NY, Guo FQ (2018). Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci 19, 849. |
| [110] | Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004). Electron transport is the functional limitation of photosynthesis in field-grown pima cotton plants at high temperature. Plant Cell Environ 27, 717-724. |
| [111] | Wu TY, Juan YT, Hsu YH, Wu SH, Liao HT, Fung RWM, Charng YY (2013). Interplay between heat shock proteins HSP101 and HSA32 prolongs heat acclimation memory posttranscriptionally in Arabidopsis. Plant Physiol 161, 2075-2084. |
| [112] | Xi YP, Han XY, Zhang ZZ, Joshi J, Borza T, Mohammad Aqa M, Zhang BB, Yuan HM, Wang-Pruski G (2020). Exogenous phosphite application alleviates the adverse effects of heat stress and improves thermotolerance of potato (Solanum tuberosum L.) seedlings. Ecotoxicol Environ Saf 190, 110048. |
| [113] | Xia ZQ, Si LY, Jin Y, Fu YF, Wang Q, Lu HD (2021). Effects of root zone temperature increase on physiological indexes and photosynthesis of different genotype maize seedlings. Russ J Plant Physiol 68, 169-178. |
| [114] | Xu HG, Liu GJ, Liu GT, Yan BF, Duan W, Wang LJ, Li SH (2014). Comparison of investigation methods of heat injury in grapevine (Vitis) and assessment to heat tolerance in different cultivars and species. BMC Plant Biol 14, 156. |
| [115] | Xu QZ, Huang BR (2001). Morphological and physiological characteristics associated with heat tolerance in creeping bentgrass. Crop Sci 41, 127-133. |
| [116] | Yamamoto H, Shikanai T (2019). PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol 179, 588-600. |
| [117] | Yamamoto Y (2016). Quality control of photosystem II: the mechanisms for avoidance and tolerance of light and heat stresses are closely linked to membrane fluidity of the thylakoids. Front Plant Sci 7, 1136. |
| [118] | Yamori N, Levine CP, Mattson NS, Yamori W (2022). Optimum root zone temperature of photosynthesis and plant growth depends on air temperature in lettuce plants. Plant Mol Biol 110, 385-395. |
| [119] | Yamori W, Hikosaka K, Way DA (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Pho- tosynth Res 119, 101-117. |
| [120] | Yamori W, Shikanai T, Makino A (2015). Photosystem I cyclic electron flow via chloroplast NADH dehydrogenase- like complex performs a physiological role for photosyn- thesis at low light. Sci Rep 5, 13908. |
| [121] | Yin YL, Qin KZ, Song XW, Zhang QH, Zhou YH, Xia XJ, Yu JQ (2018). BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase- mediated reactive oxygen species signaling in tomato. Plant Cell Physiol 59, 2239-2254. |
| [122] | Zhang HM, Zhu JH, Gong ZZ, Zhu JK (2022). Abiotic stress responses in plants. Nat Rev Genet 23, 104-119. |
| [123] | Zheng XT, Wang CJ, Lin WX, Lin CF, Han DL, Xie Q, Lai JB, Yang CW (2022). Importation of chloroplast proteins under heat stress is facilitated by their SUMO conjugations. New Phytol 235, 173-187. |
| [124] | Zhong LL, Zhou W, Wang HJ, Ding SH, Lu QT, Wen XG, Peng LW, Zhang LX, Lu CM (2013). Chloroplast small heat shock protein HSP21 interacts with plastid nucleoid protein pTAC5 and is essential for chloroplast development in Arabidopsis under heat stress. Plant Cell 25, 2925-2943. |
/
| 〈 |
|
〉 |