

收稿日期: 2020-02-05
录用日期: 2020-06-05
网络出版日期: 2020-06-05
基金资助
国家自然科学基金(31571467);齐鲁师范学院校级青年教师科研基金(2018L0402)
An Improved Protocol for Whole Mount Clearing of Plant Root Tip
Received date: 2020-02-05
Accepted date: 2020-06-05
Online published: 2020-06-05
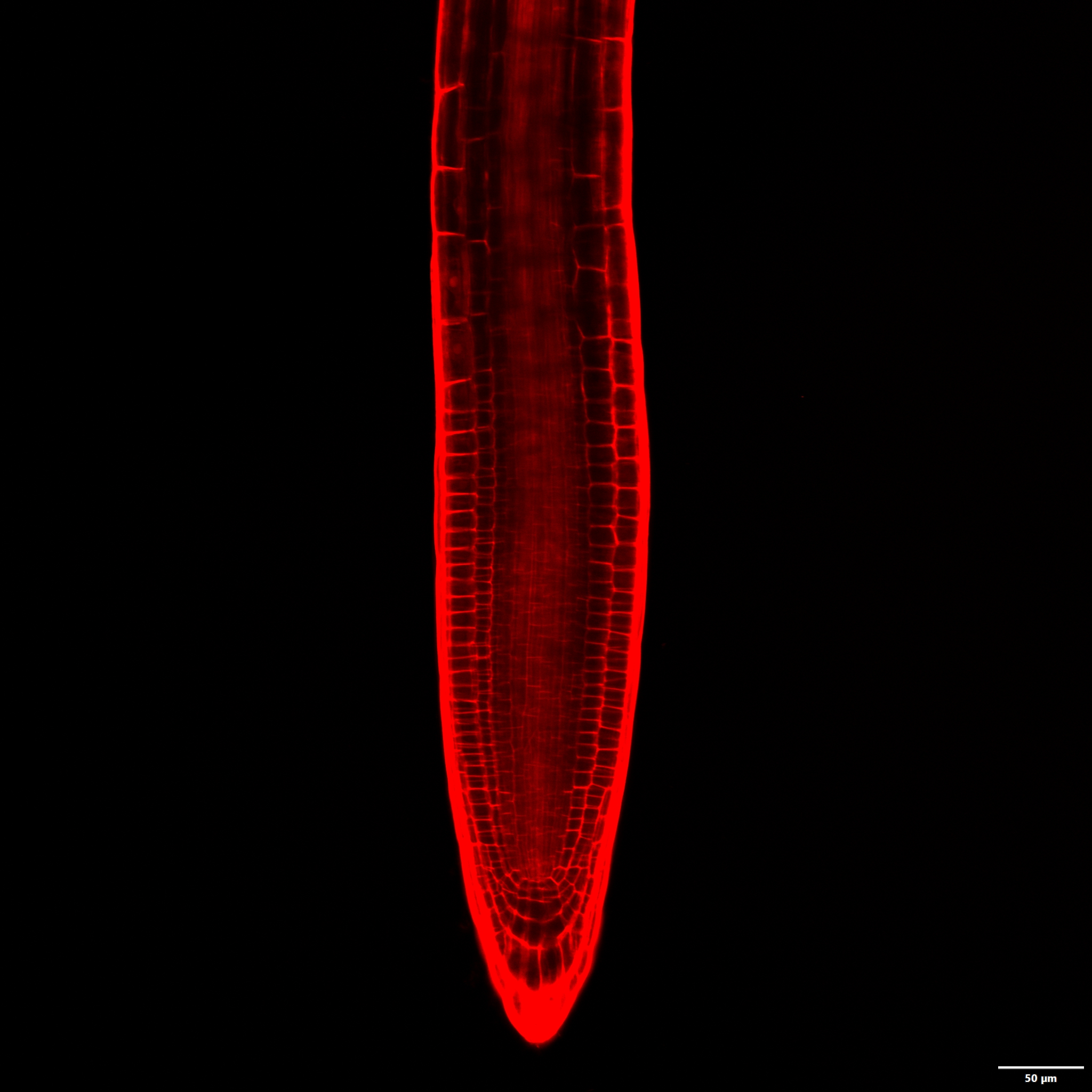
整体透明观察技术是植物形态发育研究的基础手段之一, 是无需制作切片直接观察植物体内部形态结构的有效方法。该技术采用高折射率介质降低光在样品中的散射, 提高光通量, 增加视野深度, 从而实现组织样品透明观察。然而透明剂能改变透明液的渗透势和pH值, 从而对细胞形态保持产生负面影响。目前, 针对植物叶片和胚珠已建立了相对成熟的整体透明观察体系, 但根尖由于细胞壁较薄, 现有的整体透明方法常导致细胞形态改变, 不确定性增加(如根尖整体形态改变和细胞发生严重的质壁分离)。该研究以拟南芥(Arabidopsis thaliana)幼苗为实验材料, 通过检测根尖形态、细胞质壁分离情况和细胞清晰度, 对常用的透明液组分、pH值和透明时间进行优化, 旨在建立一种适用于根尖等较脆弱组织材料的整体透明方法。
马龙 , 李桂林 , 李师鹏 , 蒋苏 . 根尖整体透明技术改良[J]. 植物学报, 2020 , 55(5) : 596 -604 . DOI: 10.11983/CBB20016
Whole mount clearing is a routine method in morphological study, which allows observation of plant internal structure without section. Using high refractive index materials as medium, clearing techniques reduce light scattering, acquire enhancive light quantity and increase depth of field and vertical planes in a particular focal plane, to facilitate the samples transparency for observation. Nevertheless, clearing materials may disturb the osmosis and pH of sample medium, which is adverse to cells morphology. So far, the effective clearing techniques have been widely used in several studies with ovule and leaf. However, the current protocol is not reliable enough for root tip clearing, because the thin cell wall is vulnerable under the treatment of clearing solutions, resulting in abnormal root tips and cells plasmolysis. To achieve a stable and optimized clearing method for root tip, we established a standard protocol via evaluation of root tip morphology, plasmolysis and cells clarity in Arabidopsis thaliana. With these improved clearing methods, we developed an optimized clearing observation system (including clearing time, pH and composition) for root tip, which could provide a reliable technique for vulnerable tissues clearing.

Key words: morphogenesis; whole mount clearing; root tip; Arabidopsis thaliana
| [1] | 程蔚玲, 丁兰, 李金平, 刘国安, 杨宁 ( 2017). Leukamenin E调节拟南芥幼苗生长发育的模式及其机制. 生态学杂志 36, 676-686. |
| [2] | 郝建华, 强胜 ( 2007). 整体透明技术在植物生物学中的应用实例及其剖析. 植物学通报 24, 490-497. |
| [3] | 李芳芳, 杨娜, 钱猛, 甘立军 ( 2018). 生长素参与三十烷醇诱导的拟南芥侧根发育. 南京农业大学学报 41, 473-480. |
| [4] | 李彦坤, 臧巩固, 赵立宁, 李育君, 唐蜻, 程超华 ( 2011). 整体透明技术观察悬铃叶苎麻胚胎发育的方法研究. 中国麻业科学 33, 142-146. |
| [5] | 任媛媛, 朱炎 ( 2017). INO80参与拟南芥气孔数量调控的分子机制. 复旦学报(自然科学版) 56, 653-661. |
| [6] | 王培新, 张丹, 尚爱加, 侯冰 ( 2016). 组织透明技术. 神经解剖学杂志 32, 124-128. |
| [7] | 王文婧, 刘婷, 郭磊, 刘春明 ( 2011). SLC/AGO1基因控制拟南芥细胞分裂与定向伸长. 植物学报 46, 370-378. |
| [8] | 杨弘远 ( 1986). 用整体染色与透明技术观察胚囊、胚、胚乳和胚状体. 植物学报 28, 575-581. |
| [9] | 杨弘远 ( 1988). 植物胚胎学中的整体透明技术. 植物学通报 5(2), 114-116. |
| [10] | 于明明, 李兴国, 张宪省 ( 2009). APETALA1启动子驱动AtIPT4在转基因拟南芥中表达导致花和花器官发育异常. 植物学报 44, 59-68. |
| [11] | 赵林姝, 刘录祥, 古佳玉, 郭会君, 李军辉, 谢永盾, 赵世荣 ( 2014). 一种小麦叶片气孔保卫细胞观察样品的制备方法. 植物学报 49, 120-126. |
| [12] | Beemster GTS, Baskin TI ( 1998). Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116, 1515-1526. |
| [13] | Bougourd S, Marrison J, Haseloff J ( 2000). An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. Plant J 24, 543-550. |
| [14] | Bruzzese E, Hasan S ( 1983). A whole leaf clearing and staining technique for host specificity studies of rust fungi. Plant Pathol 32, 335-338. |
| [15] | Crane CF ( 1978). Apomixis and Crossing Incompatibilities in Some Zephyrantheae. Ph.D. thesis. Austin: University of Texas. |
| [16] | Derbyshire P, Findlay K, McCann MC, Roberts K ( 2007). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J Exp Bot 58, 2079-2089. |
| [17] | Hager A ( 2003). Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116, 483-505. |
| [18] | Haseloff J ( 2003). Old botanical techniques for new microscopes. BioTechniques 34, 1174-1182. |
| [19] | Herr JMJ ( 1971). A new clearing-squash technique for the study of ovule development in angiosperms. Am J Bot 58, 785-790. |
| [20] | Herr JMJ (1993). Clearing techniques for the study of vascular plant tissues in whole structures and thick sections. In: Tested Studies for Laboratory Teaching. Proceedings of the Fifth Workshop/Conference of the Association for Biology Laboratory Education (ABLE). Toronto: ABLE. pp. 63-84. |
| [21] | Hoyer H ( 1882). Beitr?ge zur histologischen Technik. 3. Einschlussflüssigkeiten. Biologisches Zentralblatt 2, 23-24. |
| [22] | Itoh K, Nakamura Y, Kawata H, Yamada T, Ohta E, Sakata M ( 1987). Effect of osmotic stress on turgor pressure in mung bean root cells. Plant Cell Physiol 28, 987-994. |
| [23] | Ivanov VB, Dubrovsky JG ( 2013). Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends Plant Sci 18, 237-243. |
| [24] | Janicka-Russak M (2011). Plant plasma membrane H+- ATPase in adaptation of plants to abiotic stresses. In: Shanker A, ed. Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives. Rijeka: Intech Open Press. pp. 197-218. |
| [25] | Kim YX, Stumpf B, Sung J, Lee SJ ( 2018). The relationship between turgor pressure change and cell hydraulics of midrib parenchyma cells in the leaves of Zea mays. Cells 7, 180. |
| [26] | Kurihara D, Mizuta Y, Sato Y, Higashiyama T ( 2015). ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168-4179. |
| [27] | Lang I, Sassmann S, Schmidt B, Komis G ( 2014). Plasmolysis: loss of turgor and beyond. Plants (Basel) 3, 583-593. |
| [28] | Lersten NR ( 1986). Modified clearing method to show sieve tubes in minor veins of leaves. Stain Technol 61, 231-234. |
| [29] | Li SP, Chen M, Yu DL, Ren SC, Sun SF, Liu LD, Ketelaar T, Emons AMC, Liu CM ( 2013). EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25, 1774-1786. |
| [30] | Li SP, van Os GMA, Ren SC, Yu DL, Ketelaar T, Emons AMC, Liu CM ( 2010). Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol 154, 1819-1830. |
| [31] | Liberato JR, Barreto RW, Shivas RG ( 2005). Leaf-clearing and staining techniques for the observation of conidiophores in the Phyllactinioideae (Erysiphaceae). Australas Plant Pathol 34, 401-404. |
| [32] | Pavelescu I, Vilarrasa-Blasi J, Planas-Riverola A, González-García MP, Ca?o-Delgado AI, Iba?es M ( 2018). A Sizer model for cell differentiation in Arabidopsis thaliana root growth. Mol Syst Biol 14, e7687. |
| [33] | Richmond PA, Métraux JP, Taiz L ( 1980). Cell expansion patterns and directionality of wall mechanical properties in Nitella. Plant Physiol 65, 211-217. |
| [34] | Shabala S, Babourina O, Newman I ( 2000). Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot 51, 1243-1253. |
| [35] | Shabala SN, Lew RR ( 2002). Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129, 290-299. |
| [36] | Takatsuka H, Umeda M ( 2014). Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot 65, 2633-2643. |
| [37] | Ursache R, Andersen TG, Marhavy P, Geldner N ( 2018). A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J 93, 399-412. |
| [38] | Verbelen JP, de Cnodder T, Le J, Vissenberg K, Balu?ka F ( 2006). The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Plant Signal Behav 1, 296-304. |
| [39] | Villani TS, Koroch AR, Simon JE ( 2013). An improved clearing and mounting solution to replace chloral hydrate in microscopic applications. Appl Plant Sci 1, apps. 1300016. |
/
| 〈 |
|
〉 |