

雷公藤悬浮细胞原生质体的制备及瞬时转化体系的建立
收稿日期: 2016-08-21
录用日期: 2017-01-10
网络出版日期: 2017-01-10
基金资助
国家自然科学基金优秀青年科学基金(No.81422053)、国家杰出青年科学基金(No.81325023)和“十三五”时期北京市属高校高水平教师队伍建设支持计划(No.CIT&TCD20170324)
Protoplast Isolation and Establishment of Transient Expression System of Tripterygium wilfordii Suspension Culture Cells
Received date: 2016-08-21
Accepted date: 2017-01-10
Online published: 2017-01-10
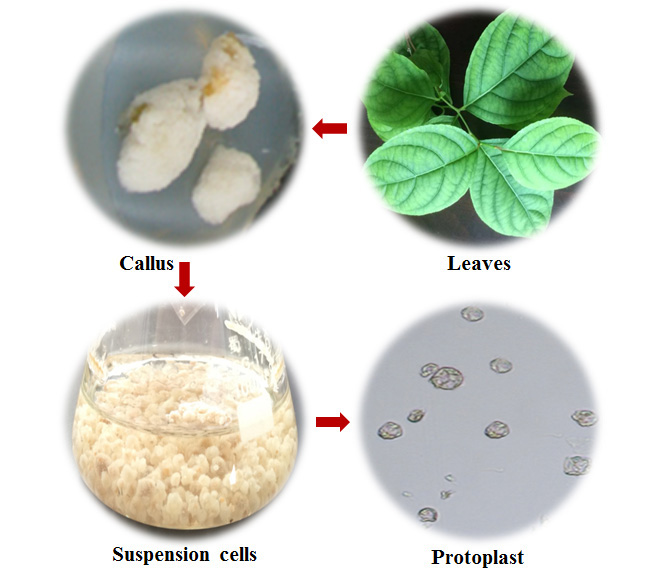
为探索药用植物雷公藤(Tripterygium wilfordii)悬浮细胞原生质体提取的最优条件, 并建立雷公藤原生质体瞬时转化体系, 以雷公藤悬浮细胞为材料, 对酶解液配比、酶解时间、甘露醇浓度及处理转速进行考察。用PEG介导的瞬时转化法将外源基因转化到雷公藤原生质体中。结果表明, 以雷公藤悬浮细胞为材料提取原生质体的最佳条件是酶液配比为2.0%纤维素酶+0.5%果胶酶+0.5%离析酶, 甘露醇浓度为0.6 mol?L-1, 酶解10小时, 处理转速为67×g; 用PEG介导法将含有编码GFP的植物表达载体转化雷公藤悬浮细胞原生质体, 激光共聚焦扫描显微镜下细胞显示绿色荧光。通过实验筛选得到雷公藤悬浮细胞原生质体的最佳提取条件, 建立了雷公藤悬浮细胞原生质体的瞬时转化体系, 为进一步开展雷公藤功能基因及合成生物学研究奠定了基础。
胡添源 , 王睿 , 陈上 , 马宝伟 , 高伟 , 黄璐琦 . 雷公藤悬浮细胞原生质体的制备及瞬时转化体系的建立[J]. 植物学报, 2017 , 52(6) : 774 -782 . DOI: 10.11983/CBB16171
We aimed to find the best protoplast extraction conditions with Tripterygium wilfordii suspension culture cells and establish an efficient transient expression system. Key factors in protoplast isolation, such as concentration of enzymes, time of enzymolysis, osmotic pressure and centrifugation speed, were studied. PEG mediation was used to transfer the GFP gene into protoplasts. The optimal extraction system was with cellulose R-10 2.0%, pectinase Y-23 0.5%, macerozyme R-10 0.5%, 0.6 mol?L-1 mannitol. The suitable enzymolysis time was 10 h, and the optimal centrifugation speed was 67 ×g. Under LSCM, the protoplast emitted green fluorescence after transforming the plasmid encoding green fluorescent protein into it. We revealed the optional conditions to isolate protoplast of T. wilfordii suspension cells and established the transient transformation system; it laid a foundation for further study on the functional genes and syn- thetic biology of T. wilfordii.

| [1] | 段炼, 钱君, 郭小雨, 朱英 (2014). 一种快速高效的水稻原生质体制备和转化方法的建立. 植物生理学报 50, 351-357. |
| [2] | 李妮娜, 丁林云, 张志远, 郭旺珍 (2014). 棉花叶肉原生质体分离及目标基因瞬时表达体系的建立. 作物学报 40, 231-239. |
| [3] | 刘凡, 赵泓, 秦帆 (2006). 结球白菜下胚轴原生质体培养及其体细胞胚植株再生. 植物学通报 23, 275-280. |
| [4] | 刘继红, 邓秀新 (1999). 植物原生质体非对称融合及其在育种上的应用. 生命科学 11, 88-91. |
| [5] | 张良波, 李培旺, 黄振, 李昌珠 (2011). 木本植物原生质体制备体系的研究进展. 中南林业科技大学学报 31(8), 102-107. |
| [6] | 朱楠, 刘俊, 张馨宇, 董娟娥 (2014). 丹参悬浮培养细胞原生质体的制备和活力检测. 生物工程学报 30, 1612-1621. |
| [7] | Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK (2012). A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med 4, 156ra139. |
| [8] | Cocking EC (1960). A method for the isolation of plant protoplasts and vacuoles.Nature 187, 927-929. |
| [9] | Duarte P, Ribeiro D, Carqueijeiro I, Bettencourt S, Sottomayor M (2016). Protoplast transformation as a plant- transferable transient expression system.Methods Mol Biol 1405, 137. |
| [10] | Gilroy S, Jones RL (1992). Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secre- tory activity in barley aleurone protoplasts.Proc Natl Acad Sci USA 89, 3591-3595. |
| [11] | Guo ZJ, Kallus S, Akiyoshi K, Sunamoto J (2006). Artificial cell wall for plant protoplast. Coating of plasma membrane with hydrophobized polysaccharides.Chem Lett 31, 415-416. |
| [12] | Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium.Nature 352, 524-526. |
| [13] | Liu JL, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U (2015). Treatment of obesity with celastrol.Cell 161, 999-1011. |
| [14] | Lu L, Li FQ, Wang XM (2010). Novel anti-inflammatory and neuroprotective agents for Parkinson’s disease.CNS Neu- rol Disord Drug Targets 9, 232-240. |
| [15] | Maas C, Werr W (1989). Mechanism and optimized conditions for PEG mediated DNA transfection into plant protoplasts.Plant Cell Rep 8, 148-151. |
| [16] | Nagata T, Takebe I (1970). Cell wall regeneration and cell division in isolated tobacco mesophyll protoplasts.Planta 92, 301-308. |
| [17] | Sheen J (2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts.Plant Physiol 127, 1466-1475. |
| [18] | Su P, Tong YR, Cheng QQ, Hu YT, Zhang M, Yang J, Teng QZ, Gao W, Huang LQ (2016). Functional characterization of ent-copalyl diphosphate synthase, kaurene synthase and kaurene oxidase in the Salvia miltiorrhiza gibberellin biosynthetic pathway. Sci Rep 6, 23057. |
| [19] | Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang YJ, Smeaton M, Demain AL, Miller PS, Kugel JF, Goodrich JA, Liu JO (2011). XPB, a subunit of TFIIH, is a target of the natural product triptolide.Nat Chem Biol 7, 182-188. |
| [20] | Tudses N, Premjet S, Premjet D (2015). Establishment of method for protoplast fusion with peg-mediated between jatropha curcas l. and ricinus communis l.Int J Life Sci Biotech Pharm Res 4, 50-56. |
| [21] | Wang X, Liang XB, Li FQ, Zhou HF, Liu XY, Wang JJ, Wang XM (2008). Therapeutic strategies for Parkinson’s disease: the ancient meets the future-traditional Chinese herbal medicine, electroacupuncture, gene therapy and stem cells.Neurochem Res 33, 1956-1963. |
| [22] | Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015). DNA-free genome editing in plants with preassembled CRISPR- Cas9 ribonucleoproteins. Nat Biotechnol 33, 1162-1164. |
| [23] | Zhang M, Su P, Zhou YJ, Wang XJ, Zhao YJ, Liu YJ, Tong YR, Hu TY, Huang LQ, Gao W (2015). Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep 34, 2179-2188. |
| [24] | Zhao YJ, Chen X, Zhang M, Su P, Liu YJ, Tong YR, Wang XJ, Huang LQ, Gao W (2015). Molecular cloning and characterisation of farnesyl pyrophosphate synthase from Tripterygium wilfordii. PLoS One 10, r0125415. |
| [25] | Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH (2012). Triptolide: structural modifications, structure-activity relations- hips, bioactivities, clinical development and mechanisms.Nat Prod Rep 29, 457-475. |
/
| 〈 |
|
〉 |