植物学报 >
2018 , Vol. 53 >Issue 6: 782 - 792
DOI:
https://doi.org/10.11983/CBB1iv>
基于孢子形态和分子证据探讨鳞盖蕨属(碗蕨科)系统分类
- 罗俊杰 ,
- 王莹 ,
- 商辉 ,
- 周喜乐 ,
- 韦宏金 ,
- 黄素楠 ,
- 顾钰峰 ,
- 金冬梅 ,
- 戴锡玲 ,
- 严岳鸿
- 1上海辰山植物园, 中国科学院上海辰山植物科学研究中心, 上海 201602
2上海师范大学生命与环境科学学院, 上海 200234
3国家林业局华东野生濒危资源植物保育中心, 上海 201602
4湘西自治州森林资源监测中心, 吉首 416000
作者简介:白克智, 1959年开始在中国科学院植物研究所工作, 先后任助理研究员、研究员, 长期从事植物生长发育及其调控的研究。1986年,其主持的“满江红生物学特性研究”荣获中国科学院科技进步二等奖。曾任《植物生理学报》编委、《植物学报》常务编委、中国植物生长调节剂协会主任等职。
收稿日期: 2017-12-29
网络出版日期: 2018-05-01
基金资助
国家自然科学基金(No.31370234)、上海市绿化和市容管理局科技攻关项目(No.G162401)和科技部科技基础性工作专项(No. 2013FY112100)
Phylogeny and Systematics of the Genus Microlepia (Dennstaedtiaceae) based on Palynology and Molecular Evidence
- Luo Junjie ,
- Wang Ying ,
- Shang Hui ,
- Zhou Xile ,
- Wei Hongjin ,
- Huang Sunan ,
- Gu Yufeng ,
- Jin Dongmei ,
- Dai Xiling ,
- Yan Yuehong
- 1Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences, Shanghai Chenshan Botanical Garden, Shanghai 201602, China
2College of Life and Environmental Sciences, Shanghai Normal University, Shanghai 200234, China
3Eastern China Conservation Center for Wild Endangered Plant Resources, State Forestry Administration, Shanghai 201602, China
4Xiangxi Autonomous Prefecture Forest Resources Monitoring Center, Jishou 416000, China
Received date: 2017-12-29
Online published: 2018-05-01
摘要
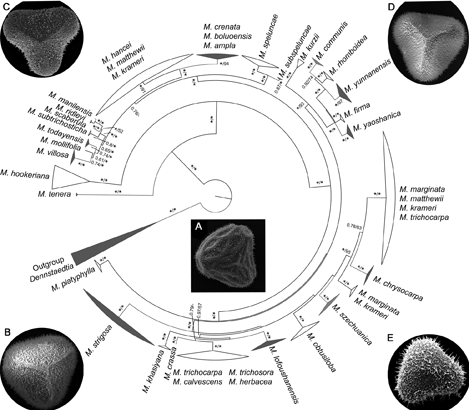
孢粉学是解决植物分类中疑难类群物种微形态分化的重要方法, 随着分子系统学的发展, 结合这两门学科的优势可以更加有效地解决疑难类群的分类学问题。鳞盖蕨属(Microlepia)是一个分类困难的疑难类群, 采用孢粉学与分子系统学一一对应的方法, 以及居群取样方式, 选取280份样本, 联合4个叶绿体片段(rbcL、trnL-F、psbA-trnH和rps4), 采用最大似然法和贝叶斯法构建该属的系统发生关系, 在此基础上对凭证标本中100份材料的孢子进行观察和分析。综合分子系统学和孢粉学的研究结果, 得出结论: (1) 在形态学研究中广泛被接受的15个物种得到了单系支持, 并厘清了分类困难的复合群; (2) 发现边缘鳞盖蕨(M. marginata)可能存在隐性种; (3) 建议恢复过去归并处理为异名的瑶山鳞盖蕨(M. yaoshanica)、罗浮鳞盖蕨(M. lofoushanensis)、四川鳞盖蕨(M. szechuanica)以及滇西鳞盖蕨(M. subspeluncae); (4) 提出鳞盖蕨属可能存在杂交现象; (5) 提出鳞盖蕨属完整的属下分类建议。
本文引用格式
罗俊杰 , 王莹 , 商辉 , 周喜乐 , 韦宏金 , 黄素楠 , 顾钰峰 , 金冬梅 , 戴锡玲 , 严岳鸿 . 基于孢子形态和分子证据探讨鳞盖蕨属(碗蕨科)系统分类[J]. 植物学报, 2018 , 53(6) : 782 -792 . DOI: 10.11983/CBB17258
Abstract
Palynology is an important method to solve the micro-morphological differentiation of species in some complex groups. With the development of molecular phylogeny, combining the advantages of these two subjects may effectively solve the taxonomic issues in plants. Microlepia (Dennstaedtiaceae) is one of the most difficult genera in ferns in terms of taxonomy. In the present study, based on palynology matched with phylogeny and population sampling, we constructed the phylogeny of 280 samples by using both Maximum Likelihood and Bayesian methods with four plastid markers (rbcL, trnL-F, psbA-trnH and rps4). The spore morphology of 100 samples was observed and analyzed. Our results of comprehensive molecular phylogeny and palynology showed that (1) 15 species widely accepted based on plant morphology were strongly supported, and this complex group of Microlepia has been further clarified; (2) There may be crytic species in the populations of M. marginata; (3) M. yaoshanica Ching, M. lofoushanensis Ching, M. szechuanica Ching and M. subspeluncae Ching, treated as synonyms in previous studies, should be restored as independent species; (4) Many hybridization events of Microlepia were found; and (5) A complete infrageneric taxonomy of Microlepia was proposed.

Key words: crytic species; hybrids; palynology; phylogeny and systematics; pteridophytes; taxonomy
参考文献
| [1] | 曹建国, 于晶, 王全喜 (2007). 中国蕨类植物孢子的形态VII. 桫椤科. 云南植物研究 29, 7-12. |
| [2] | 陈焕镛 (1964). 海南植物志, 第1卷. 北京: 科学出版社. pp. 46-52. |
| [3] | 广西植物研究所 (2013). 广西植物志. 南宁: 广西科学技术出版社. pp. 96-108. |
| [4] | 孔宪需 (1988). 四川植物志, 第6卷. 蕨类植物门. 成都: 四川科学技术出版社. pp. 183-190. |
| [5] | 刘红梅, 张宪春, 曾辉 (2009). DNA序列在蕨类分子系统学研究中的应用. 植物学报 44, 143-158. |
| [6] | 牟善杰 (2010). 碗蕨科鳞盖蕨属专论研究. 博士论文. 台北: 台湾师范大学. pp. 1-198. |
| [7] | 秦仁昌 (1959). 中国植物志, 第2卷. 北京: 科学出版社. pp. 207-246. |
| [8] | 王培善, 王筱英 (2001). 贵州蕨类植物志. 贵阳: 贵州科技出版社. pp. 433-442. |
| [9] | 王全喜, 陈立群, 包文美 (1997). 中国金毛裸蕨属植物孢子形态的研究. 西北植物学报 17(5), 44-47. |
| [10] | 王全喜, 戴锡玲 (2010). 中国水龙骨目(真蕨目)植物孢子形态的研究. 北京: 科学出版社. pp. 1-25. |
| [11] | 王全喜, 于晶 (2003). 扫描电镜下真蕨目孢子表面纹饰的分类. 云南植物研究 25, 313-320. |
| [12] | 吴德邻 (2006). 广东植物志, 第7卷. 广州: 广东科技出版社. pp. 81-85. |
| [13] | 吴征镒 (2006). 云南植物志, 第12卷. 北京: 科学出版社. pp. 216-230. |
| [14] | 杨鲁红 (2012). 中国石韦属植物系统分类学研究. 硕士论文. 昆明: 云南大学. pp. 1-142. |
| [15] | 张宪春, 卫然, 刘红梅, 何丽娟, 王丽, 张钢民 (2013). 中国现代石松类和蕨类的系统发育与分类系统. 植物学报 48, 119-137. |
| [16] | 张玉龙, 席以珍, 张金谈, 高桂珍, 杜乃秋, 孙湘君, 孔昭宸 (1976). 中国蕨类植物孢子形态. 北京: 科学出版社. pp. 1-112. |
| [17] | Akaike H (1974). A new look at the statistical model identification.IEEE Trans Automat Contr 19, 716-723. |
| [18] | Burland TG (2000). Dnastar’s lasergene sequence analysis software. In: Misener S, Krawetz SA, eds. Bioinformatics Methods and Protocols. Methods in Molecular BiologyTM, Vol. 132. Totowa, NJ: Humana Press. pp. 71-91. |
| [19] | Denk T, Grimm GW (2009). Significance of pollen characteristics for infrageneric classification and phylogeny in Quercus(Fagaceae). Int J Plant Sci 170, 926-940. |
| [20] | Ebihara A, Nitta JH, Ito M (2010). Molecular species identification with rich floristic sampling: DNA barcoding the pteridophyte flora of Japan.PLoS One 5, e15136. |
| [21] | Hall TA (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT.Nucl Acids Sym Ser 41, 95-98. |
| [22] | Hasebe M, Omori T, Nakazawa M, Sano T, Kato M, Iwatsuki K (1994). rbcL gene sequences provide evidence for the evolutionary lineages of leptosporangiate ferns. Proc Natl Acad Sci USA 91, 5730-5734. |
| [23] | Huang TC (1981). Spore Flora of Taiwan (Pteridophyta). Taiwan: National Taiwan University. pp. 52-53. |
| [24] | Kramer KU (1990). Dennstaedtiaceae. In: Kramer KU, Green PS, eds. The Families and Genera of Vascular Plants, Vol. I. Pteridophytes and Gymnosperms. Berlin: Springer-Verlag. pp. 81-94. |
| [25] | Lehtonen S, Wahlberg N, Christenhusz MJM (2012). Diversification of lindsaeoid ferns and phylogenetic uncertainty of early polypod relationships.Bot J Linn Soc 170, 489-503. |
| [26] | Li FW, Kuo LY, Rothfels CJ, Ebihara A, Chiou WL, Windham MD, Pryer KM (2011). rbcL and matK earn two thumbs up as the core DNA barcode for ferns. PLoS One 6, e26597. |
| [27] | Miller MA, Pfeiffer W, Schwartz T (2010). Creating the cipres science gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop (GCE). New Orleans: IEEE. pp. 1-8. |
| [28] | Moran RC, Hanks JG, Rouhan G (2007). Spore morpho- logy in relation to phylogeny in the fern genus Elaphoglossum(Dryopteridaceae). Int J Plant Sci 168, 905-929. |
| [29] | Nakato N, Ebihara A (2011). Chromosome number of Microlepia hookeriana (Dennstaedtiaceae) and chromosome number evolution in the genus Microlepia. Bull Natl Mus Nat Sci Ser B 37, 75-78. |
| [30] | Posada D (2008). Jmodeltest: phylogenetic model averaging.Mol Biol Evol 25, 1253-1256. |
| [31] | Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R (2004). Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences.Am J Bot 91, 1582-1598. |
| [32] | Pryer KM, Smith AR, Hunt JS, Dubuisson JY (2001). rbcL data reveal two monophyletic groups of filmy ferns(Filicopsida: Hymenophyllaceae). Am J Bot 88, 1118-1130. |
| [33] | Rambaut A, Drummond AJ (2007). Tracer v1.4. . |
| [34] | Ronquist F, Huelsenbeck JP (2003). Mrbayes 3: Bayesian phylogenetic inference under mixed models.Bioinforma- tics 19, 1572-1574. |
| [35] | Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space.Syst Biol 61, 539-542. |
| [36] | Schuettpelz E, Korall P, Pryer KM (2006). Plastid atpA data provide improved support for deep relationships among ferns. Taxon 55, 897-906. |
| [37] | Schuettpelz E, Pryer KM (2007). Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes.Taxon 56, 1037-1050. |
| [38] | Schwarz G (1978). Estimating the dimension of a model.Ann Statist 6, 461-464. |
| [39] | Shaw J, Lickey EB, Beck JT, Farmer SB, Liu WS, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL (2005). The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis.Am J Bot 92, 142-166. |
| [40] | Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2006). A classification for extant ferns.Taxon 55, 705-731. |
| [41] | Souza-Chies TT, Bittar G, Nadot S, Carter L, Besin E, Lejeune B (1997). Phylogenetic analysis of Iridaceae with parsimony and distance methods using the plastid gene rps4. Plant Syst Evol 204, 109-123. |
| [42] | Swofford DL (2003). Paup*: Phylogenetic Analysis Using Parsimony, Version 4.0 b10. |
| [43] | Taberlet P, Gielly L, Pautou G, Bouvet J (1991). Universal primers for amplification of three non-coding regions of chloroplast DNA.Plant Mol Biol 17, 1105-1109. |
| [44] | Tagawa M (1952). Fern miscellany (6).J Jpn Bot 27, 213-218. |
| [45] | Tate JA, Simpson BB (2003). Paraphyly of Tarasa(Malvaceae) and diverse origins of the polyploid species. Syst Bot 28, 723-737. |
| [46] | The Pteridophyte Phylogeny Group (2016). A community-derived classification for extant lycophytes and ferns.J Syst Evol 54, 563-603. |
| [47] | Tryon AF, Lugardon B (1991). Spores of the Pteridophyta. Berlin: Springer-Verlag. pp. 1-279. |
| [48] | Wang FG, Liu HM, He CM, Yang DM, Xing FW (2015). Taxonomic and evolutionary implications of spore ornamentation in Davalliaceae.J Syst Evol 53, 72-81. |
| [49] | Wolf PG (1995). Phylogenetic analyses of rbcL and nuclear ribosomal RNA gene sequences in Dennstaedtiaceae. Am Fern J 85, 306-327. |
| [50] | Wolf PG (1997). Evaluation of atpB nucleotide sequences for phylogenetic studies of ferns and other pteridophytes. Am J Bot 84, 1429-1440. |
| [51] | Xu Y, Hu CM, Hao G (2016). Pollen morphology of Androsace(Primulaceae) and its systematic implications. J Syst Evol 54, 48-64. |
| [52] | Yan YH, Qi XP, Zhang XC (2013). Dennstaedtiaceae. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Vol. 2-3. Pteridophytes. Beijing: Science Press; St. Louis: Missouri Botanical Garden Press. pp. 147-168. |
| [53] | Yanez A, Marquez GJ, Morbelli MA (2016). Palynological analysis of Dennstaedtiaceae taxa from the paranaense phytogeographic province that produce trilete spores II: Microlepia speluncae and Pteridium arachnoideum. An Acad Bras Ciênc 88, 877-890. |
| [54] | Yuan Y, Fu L, Ma CY (2012). Microlepia boluoensis sp. nov.(Dennsteadtiaceae) from Guangdong, China. Nord J Bot 30, 168-173. |
| [55] | Zhou XM, Zhang LB (2015). A classification of Selaginella(Selaginellaceae) based on molecular (chloroplast and nuclear), macromorphological, and spore features. Taxon 64, 1117-1140. |
/
| 〈 |
|
〉 |