

苯酚废水对垂柳叶片光合生理参数的影响
收稿日期: 2015-02-03
录用日期: 2015-07-06
网络出版日期: 2016-02-01
基金资助
基金项目: 林业公益性行业科研专项(No.201404030108)、国家自然科学基金(No.31170662)、山东省水利重大专项(No;SDSLKY201231) 和山东省自然科学基金联合专项(NoZR2015CL044)
The Effect of Phenol Concentration on Photosynthetic Physiological Parameters of Salix babylonica
Received date: 2015-02-03
Accepted date: 2015-07-06
Online published: 2016-02-01
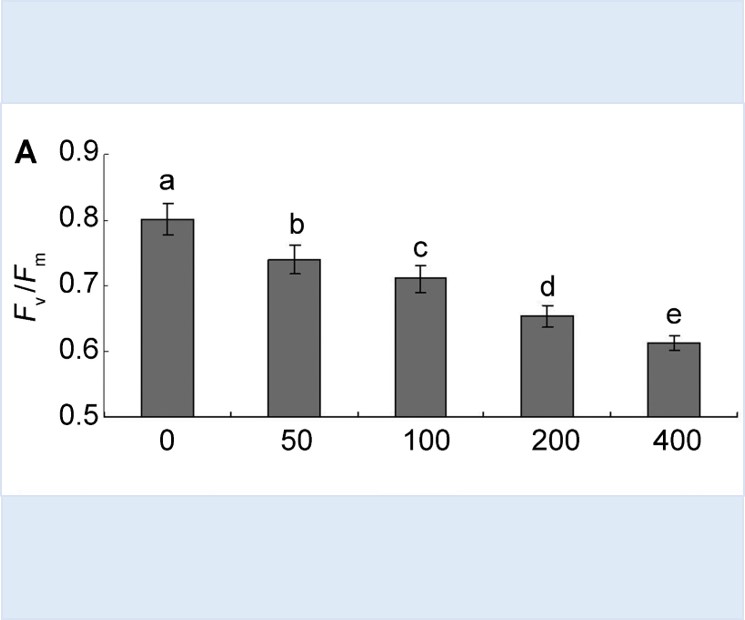
为探讨垂柳(Salix babylonica)对苯酚污染物的耐受程度及其应用于苯酚污染环境修复的可行性, 了解苯酚胁迫对垂柳光合作用生理过程的影响与限制机理, 采用水培模拟胁迫实验方法, 在5种苯酚溶液浓度(50、100、200、400和800 mg·L-1)下, 测定垂柳植株叶片光合气体交换及叶绿素荧光参数。结果表明, 苯酚对垂柳光合作用具有显著的抑制作用, 表现为叶片净光合速率(Pn)、最大光合速率(Pnmax)、光合量子效率(Φ)、PSII最大和实际光化学效率(Fv/Fm和ФPSII)等均明显下降。苯酚胁迫浓度越高, 对垂柳光合作用的抑制程度越大; 苯酚胁迫限制光合作用主要由非气孔因素引起。将垂柳用于苯酚污染的水体环境修复时, 苯酚浓度应在200 mg·L-1以下, 否则垂柳的光合作用效能会明显降低。垂柳光合作用生理活性耐受苯酚胁迫的极限浓度还需进一步实验研究。
李辉, 张光灿, 谢会成, 许景伟, 李传荣, 孙居文 . 苯酚废水对垂柳叶片光合生理参数的影响[J]. 植物学报, 2016 , 51(1) : 31 -39 . DOI: 10.11983/CBB15028
To explore the phenol tolerance of Salix babylonica and the feasibility of its remediation of phenol pollution, we determined the effect of phenol stress on photosynthetic physiology of S. babylonica and its limit mechanism, photosynthesis and chlorophyll fluorescence parameters of cut seedlings of S. babylonica in five phenol concentrations (50, 100, 200, 400 and 800 mg·L-1) using hydroponics. Phenol inhibited the photosynthesis of S. babylonica significantly, which presented as significantly decreased net photosynthesis rate (Pn), maximum net photosynthesis rate (Pnmax), photosynthetic quantum efficiency, maximum quantum yield of PS II (Fv/Fm) and actual photochemical efficiency (ФPSII). The higher the concentration of phenol, the greater the photosynthetic inhibition. Non-stomatal limitation was responsible for reduced net photosynthesis rate (Pn). When S. babylonica was used to phytoremediate the water environment, the concentration of phenol should be < 200 mg·L-1. Otherwise the efficiency of photosynthesis of S. babylonica was markedly decreased. The limit of phenol concentration that photosynthetic physiological activity of S. babylonica can tolerate needs further experimental study.

| 1 | 陈彩虹, 刘治昆, 陈光才, 单奇华, 张建锋 (2011). 苏柳172和垂柳对Cu2+的吸收特性及有机酸影响. 生态学报 31, 5255-5263. |
| 2 | 陈登举, 高培军, 吴兴波, 高岩, 温国胜, 王玉魁, 高荣孚, 张汝民 (2013). 毛竹茎秆叶绿体超微结构及其发射荧光光谱特征. 植物学报 48, 635-642. |
| 3 | 陈华新, 陈玮, 姜闯道, 高辉远, 邹琦 (2008). 光温交叉处理对小麦紫黄质脱环氧化酶活性及其热耗散能力的影响. 植物生态学报 32, 1015-1022. |
| 4 | 陈卫英, 陈真勇, 罗辅燕, 彭正松, 余懋群 (2012). 光响应曲线的指数改进模型与常用模型比较. 植物生态学报 36, 1277-1285. |
| 5 | 陈志成, 王荣荣, 王志伟, 杨吉华, 王华田, 耿兵, 张永涛 (2012). 不同土壤水分条件下栾树光合作用的光响应. 中国水土保持科学 10, 105-110. |
| 6 | 房娟, 楼崇, 陈光才, 单奇华, 张建锋 (2011). 苏柳172和垂柳对Pb的吸收动力学特性及有机酸的影响. 环境化学 30, 1569-1575. |
| 7 | 韩刚, 赵忠 (2010). 不同土壤水分下4种沙生灌木的光合光响应特性. 生态学报 30, 4019-4026. |
| 8 | 姜闯道 (2003). 高等植物光合作用中的激发能分配及光破坏防御机制. 博士论文. 泰安: 山东农业大学. pp. 41-91 |
| 9 | 江月玲 (1997). 水体酚类化合物污染对水稻幼苗生长的影响. 植物学通报 14, 41-44. |
| 10 | 郎莹, 张光灿, 张征坤, 刘顺生, 刘德虎, 胡小兰 (2011). 不同土壤水分下山杏光合作用光响应过程及其模拟. 生态学报 31, 4499-4508. |
| 11 | 梁芳, 郑成淑, 孙宪芝, 王文莉 (2010). 低温弱光胁迫及恢复对切花菊光合作用和叶绿素荧光参数的影响. 应用生态学报 21, 29-35. |
| 12 | 刘春英, 陈大印, 盖树鹏, 张玉喜, 郑国生 (2012). 高、低温胁迫对牡丹叶片PSII功能和生理特性的影响. 应用生态学报 23, 133-139. |
| 13 | 刘琼玉, 李太友 (2002). 含酚废水的无害化处理技术进展. 环境污染治理技术与设备 3, 62-64. |
| 14 | 钱永强, 周晓星, 韩蕾, 孙振元, 巨关升 (2011). Cd2+胁迫对银芽柳PSII叶绿素荧光光响应曲线的影响. 生态学报 31, 6134-6142. |
| 15 | 苏行, 胡迪琴, 林植芳, 林桂珠, 孔国辉, 彭长连 (2002). 广州市大气污染对两种绿化植物叶绿素荧光特性的影响. 植物生态学报 26, 599-604. |
| 16 | 王利, 杨洪强, 范伟国, 张召 (2010). 平邑甜茶叶片光合速率及叶绿素荧光参数对氯化镉处理的响应. 中国农业科学 43, 3176-3183. |
| 17 | 王宇明, 蔡焕杰, 王健 (2010). 冬小麦辣椒间套作对光合有效辐射和地温的影响. 中国农村水利水电 (1), 14-16. |
| 18 | 许大全 (2002). 光合作用效率. 上海: 上海科学技术出版社. pp. 29-36. |
| 19 | 杨卫东, 陈益 (2009). 垂柳对镉吸收、积累与耐性的特点分析. 南京林业大学学报(自然科学版) 33, 17-20. |
| 20 | 叶子飘, 于强 (2007). 一个光合作用光响应新模型与传统模型的比较. 沈阳农业大学学报 38, 771-775. |
| 21 | 朱英华, 屠乃美, 肖汉乾, 张国 (2011). 硫对成熟期烤烟叶绿素荧光参数的影响. 生态学报 31, 3796-3801. |
| 22 | Berry JA, Downton WJS (1982). Environmental regulation of photosynthesis. In: Govindjee, ed. Photosynthesis, Vol.II. New York: Academic Press. |
| 23 | Brack W, Frank H (1998). Chlorophyll a fluorescence: a tool for the investigation of toxic effects in the photosynthetic apparatus.Ecotox Environ Safe 40, 34-41. |
| 24 | El-Hassani FZ, Amraoui MB, Zinedine H, Aissam H, Mdaghri AS, Merzouki M, Benlemlih M (2009). Changes in leaf phenols and other physiological parameters of pep- permint in response to olive mill wastewater application.Int J Agric Biol 11, 413-418. |
| 25 | Farquhar GD, Sharkey TD (1982). Stomatal conductance and photosynthesis.Annu Rev Plant Physiol 33, 317-345. |
| 26 | Greger M, Landberg T (1999). Use of willow in phytoextraction.Int J Phytoremediat 1, 115-123. |
| 27 | Krause GH, Weis E (1991). Chlorophyll fluorescence and photosynthesis: the basics.Annu Rev Plant Biol 42, 313-349. |
| 28 | Mary LG, David ES (2001). Fortified foods and phytoremediation. Two sides of the same coin. Plant Physiol 125, 164-167. |
| 29 | Mirck J, Isebrands JG, Verwijst T, Ledin S (2005). Development of short-rotation willow coppice system for environmental purposes in Sweden.Biomass Bioenerg 28, 219-228. |
| 30 | Nijs I, Ferris R, Blum H (1997). Stomatal regulation in a changing climate: a field study using free air temperature increase (FATI) and free air CO2 enrichment (FACE). Plant Cell Environ 20, 1041-1050. |
| 31 | Oliver S, Scragg AH, Morrison J (2003). The effect of chlorophenols on the growth of Chlorella VT-1.Enzyme Microb Tech 32, 837-842. |
| 32 | Ouzounidou G, Asfi M, Sortirakis N, Papadopoulou P, Gaitisa F (2008). Olive mill wastewater triggered changes in physiology and nutritional quality of tomato (Lycopersicon esculentum Mill.) depending on growth substrate.J Hazard Mater 158, 523-530. |
| 33 | Pinol R, Simón E (2009). Effect of 24-epibrassinolide on chlorophyll fluorescence and photosynthetic CO2 assimilation in Vicia faba plants treated with the photosynthesis-inhibiting herbicide terbutryn.J Plant Growth Regul 28, 97-105. |
| 34 | Punshon T, Dickinson N (1999). Heavy metal resistance and accumulation characteristics in willows.Int J Phytoremediat 1, 361-385. |
| 35 | Quan X, Shi H, Zhang Y, Wang J, Qian Y (2004). Biodegradation of 2,4-dichlorophenol and phenol in an airlift inner-loop bioreactor immobilized with Achromobacter sp.Sep Purif Technol 34, 97-103. |
| 36 | Richard BM (2000). Phytoremediation of toxic elemental and organic pollutants.Curr Opin Plant Biol 3, 153-162. |
| 37 | Scragg AH, Spiller L, Morrison J (2003). The effect of 2,4-dichlorophenol on the microalga Chlorella VT-1.Enzyme Microb Tech 32, 616-622. |
| 38 | Susarla S, Medina V, McCutcheon S (2002). Phytoremediation: an ecological solution to organic chemical contamination.Ecol Eng 18, 647-658. |
| 39 | Ucisik AS, Trapp S (2006). Uptake, removal, accumulation, and phytotoxicity of phenol in willow trees (Salix viminalis).Environ Toxicol Chem 25, 2455-2460. |
| 40 | Yu XZ, Trapp S, Zhou PH (2005). Phytotoxicity of cyanide to weeping willow trees.Environ Sci Pollut R 12, 109-113. |
| 41 | Yulla AK, Martin F (2005). Willows beyond wetland: use of Salix L. species for environmental projects.Water Air Soil Poll 162, 183-204. |
/
| 〈 |
|
〉 |