

植物学报 ›› 2022, Vol. 57 ›› Issue (4): 412-421.DOI: 10.11983/CBB22016 cstr: 32102.14.CBB22016
吴楠1,2, 覃磊1,2, 彭志红1, 夏石头1,2,*( )
)
收稿日期:2022-01-16
接受日期:2022-04-24
出版日期:2022-07-01
发布日期:2022-07-14
通讯作者:
夏石头
作者简介:* E-mail: xstone0505@hunau.edu.cn基金资助:
Wu Nan1,2, Qin Lei1,2, Peng Zhihong1, Xia Shitou1,2,*( )
)
Received:2022-01-16
Accepted:2022-04-24
Online:2022-07-01
Published:2022-07-14
Contact:
Xia Shitou
摘要: 系统获得性抗性(SAR)是一种因病原微生物初次侵染植物局部叶片而被激活的整株水平上的持久广谱抗性。在初次侵染部位快速产生的抗性信号, 可通过韧皮部传输到植物其它部位, 从而激活SAR。哌啶酸/N-羟基哌啶酸(Pip/NHP)作为新发现的移动信号分子, 在SAR信号通路中具有重要作用。该文综述了Pip/NHP的合成、转运以及对SAR调控作用的最新研究进展。
吴楠, 覃磊, 彭志红, 夏石头. 系统获得性抗性移动信号Pip/NHP研究进展. 植物学报, 2022, 57(4): 412-421.
Wu Nan, Qin Lei, Peng Zhihong, Xia Shitou. Research Progress of Mobile Signal Pip/NHP in Systemic Acquired Resistance. Chinese Bulletin of Botany, 2022, 57(4): 412-421.
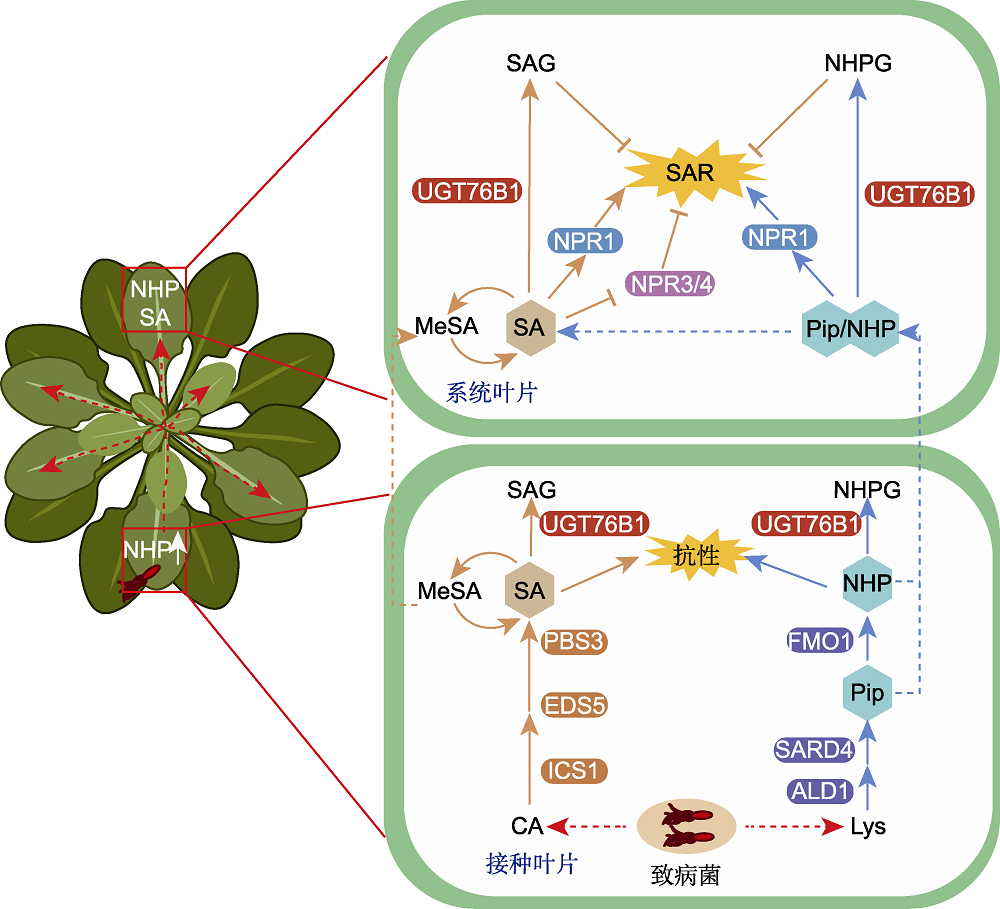
图1 哌啶酸(Pip)/N-羟基哌啶酸(NHP)的生物合成途径及其对系统获得性抗性(SAR)的调控作用 图示病原体诱导的SAR建立过程中的调控网络。NHP与水杨酸(SA)共同驱动主要的SAR诱导途径。SA由ICS1、EDS5和PBS3从分支酸(CA)开始合成, Pip由ALD1和SARD4从赖氨酸(Lys)开始合成, 再通过FMO1进一步合成NHP。Pip/NHP可通过韧皮部转运到远端叶片, 而SA通过SA甲基转移酶(MeSA)的形式转移到远端叶片。因此, 系统叶组织中的NHP水平开始升高, 从而启动NPR1依赖的转录SAR反应, 且NHP水平升高优先于SA, 因此NHP还能通过间接上调SA的方式激活SAR反应。此外, NHP/SA诱导的转录反应通过增加UGT76B1和其它分解代谢酶的活性, 将SA和NHP转化成葡糖基水杨酸(SAG)和NHP-N-O-葡萄糖苷(NHPG), 从而降低SAR活性代谢物NHP和SA的水平, 在系统水平上降低或终止SAR反应。棕色路线代表SA途径; 蓝色路线代表Pip/NHP途径; T型箭头表示抑制作用; 棕色和蓝色虚线表示长距离运输; 红色虚线表示病原菌的诱导。
Figure 1 Biosynthetic pathway of pipecolic acid (Pip)/N-hydroxy-pipecolic acid (NHP) and its regulation in systemic acquired resistance (SAR) Figure shows a schematic diagram of regulatory network in the process of pathogen induced SAR establishment. NHP and SA jointly drives the main SAR induction pathway. While SA is synthesized from chorismic acid (CA) by ICS1, EDS5 and PBS3, Pip is synthesized from Lys by ALD1 and SARD4, and then NHP is further synthesized by FMO1. Pip/NHP can be transported to distal leaves through phloem, while SA can be transferred to distal leaves in the form of MeSA. Therefore, the level of NHP in leaf tissue of the system begins to rise, which starts the NPR1 dependent transcriptional SAR response, and the increase of NHP level takes priority with SA, which means, NHP can also activate the SAR response by indirectly upregulating SA. In addition, NHP/SA induced transcriptional response converts SA and NHP into SA-O-b-glucoside (SAG) and NHP-N-O-glucoside (NHPG) by increasing the activities of UGT76B1 and other catabolic enzymes, thereby reducing the levels of SAR active metabolites NHP and SA and reducing or terminating SAR response at the system level. Brown route represents SA pathway; Blue route represents Pip/NHP pathway; T-shaped arrow indicates inhibition; Brown and blue dotted lines indicate long-distance transportation; Red dotted line indicates the induction of pathogen.
| [1] |
Attaran E, Zeier TE, Griebel T, Zeier J (2009). Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21, 954-971.
DOI PMID |
| [2] |
Bauer S, Mekonnen DW, Hartmann M, Yildiz I, Janowski R, Lange B, Geist B, Zeier J, Schäffner AR (2021). UGT76B1, a promiscuous hub of small molecule-based immune signaling, glucosylates N-hydroxypipecolic acid, and balances plant immunity. Plant Cell 33, 714-734.
DOI URL |
| [3] |
Bernsdorff F, Döring AC, Gruner K, Schuck S, Bräutigam A, Zeier J (2016). Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and -independent pathways. Plant Cell 28, 102-129.
DOI URL |
| [4] |
Bohlmann J, Keeling CI (2008). Terpenoid biomaterials. Plant J 54, 656-669.
DOI URL |
| [5] |
Cai JH, Jozwiak A, Holoidovsky L, Meijler MM, Meir S, Rogachev I, Aharoni A (2021). Glycosylation of N-hydroxy-pipecolic acid equilibrates between systemic acquired resistance response and plant growth. Mol Plant 14, 440-455.
DOI URL |
| [6] |
Chanda B, Xia Y, Mandal MK, Yu KS, Sekine KT, Gao QM, Selote D, Hu YL, Stromberg A, Navarre D, Kachroo A, Kachroo P (2011). Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet 43, 421-427.
DOI URL |
| [7] |
Chaturvedi R, Giri M, Chowdhury Z, Venables BJ, Mohanty D, Petros RA, Shah J (2020). CYP720A1 function in roots is required for flowering time and systemic acquired resistance in the foliage of Arabidopsis. J Exp Bot 71, 6612-6622.
DOI PMID |
| [8] |
Chaturvedi R, Venables B, Petros RA, Nalam V, Li MY, Wang XM, Takemoto LJ, Shah J (2012). An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J 71, 161-172.
DOI URL |
| [9] | Chen YC, Holmes EC, Rajniak J, Kim JG, Tang S, Fischer CR, Mudgett MB, Sattely ES (2018). N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc Natl Acad Sci USA 115, E4920-E4929. |
| [10] |
Delaney TP, Friedrich L, Ryals JA (1995). Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA 92, 6602-6606.
DOI URL |
| [11] |
Dempsey DA, Klessig DF (2012). SOS——too many signals for systemic acquired resistance? Trends Plant Sci 17, 538-545.
DOI URL |
| [12] |
Ding PT, Rekhter D, Ding YL, Feussner K, Busta L, Haroth S, Xu SH, Li X, Jetter R, Feussner I, Zhang YL (2016). Characterization of a pipecolic acid biosynthesis pathway required for systemic acquired resistance. Plant Cell 28, 2603-2615.
DOI URL |
| [13] |
Ding YL, Sun TJ, Ao K, Peng YJ, Zhang YX, Li X, Zhang YL (2018). Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454-1467.
DOI URL |
| [14] |
Ding YZ, Dommel MR, Wang CG, Li Q, Zhao Q, Zhang XD, Dai SJ, Mou ZL (2020). Differential quantitative requirements for NPR1 between basal immunity and systemic acquired resistance in Arabidopsis thaliana. Front Plant Sci 11, 570422.
DOI URL |
| [15] |
Durrant WE, Dong X (2004). Systemic acquired resistance. Annu Rev Phytopathol 42, 185-209.
PMID |
| [16] |
Fu ZQ, Dong XN (2013). Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64, 839-863.
DOI URL |
| [17] |
Guerra T, Schilling S, Hake K, Gorzolka K, Sylvester FP, Conrads B, Westermann B, Romeis T (2020). Calcium-dependent protein kinase 5 links calcium signaling with N-hydroxy-L-pipecolic acid- and SARD1-dependent immune memory in systemic acquired resistance. New Phytol 225, 310-325.
DOI URL |
| [18] |
Gupta RN, Spenser ID (1969). Biosynthesis of the piperidine nucleus: the mode of incorporation of lysine into pipecolic acid and into piperidine alkaloids. J Biol Chem 244, 88-94.
PMID |
| [19] |
Hartmann M, Kim D, Bernsdorff F, Ajami-Rashidi Z, Scholten N, Schreiber S, Zeier T, Schuck S, Reichel-Deland V, Zeier J (2017). Biochemical principles and functional aspects of pipecolic acid biosynthesis in plant immunity. Plant Physiol 174, 124-153.
DOI PMID |
| [20] |
Hartmann M, Zeier J (2018). L-lysine metabolism to N-hydroxypipecolic acid: an integral immune-activating pathway in plants. Plant J 96, 5-21.
DOI URL |
| [21] |
Hartmann M, Zeier J (2019). N-hydroxypipecolic acid and salicylic acid: a metabolic duo for systemic acquired resistance. Curr Opin Plant Biol 50, 44-57.
DOI PMID |
| [22] |
Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Bräutigam A, Hölzel T, Ganter C, Zeier J (2018). Flavin monooxygenase-generated N-Hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173, 456-469.
DOI PMID |
| [23] |
Holmes EC, Chen YC, Mudgett MB, Sattely ES (2021). Arabidopsis UGT76B1 glycosylates N-hydroxy-pipecolic acid and inactivates systemic acquired resistance in tomato. Plant Cell 33, 750-765.
DOI URL |
| [24] | Holmes EC, Chen YC, Sattely ES, Mudgett MB (2019). An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci Signal 12, eaay3066. |
| [25] |
Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009). Priming in systemic plant immunity. Science 324, 89-91.
DOI PMID |
| [26] |
Kim Y, Gilmour SJ, Chao L, Park S, Thomashow MF (2020). Arabidopsis CAMTA transcription factors regulate pipecolic acid biosynthesis and priming of immunity genes. Mol Plant 13, 157-168.
DOI URL |
| [27] |
Kvenvolden KA, Lawless JG, Ponnamperuma C (1971). Nonprotein amino acids in the murchison meteorite. Proc Natl Acad Sci USA 68, 486-490.
DOI URL |
| [28] |
Lim GH, Shine MB, de Lorenzo L, Yu KS, Cui WE, Navarre D, Hunt AG, Lee JY, Kachroo A, Kachroo P (2016). Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe 19, 541-549.
DOI URL |
| [29] |
Liu YN, Sun TJ, Sun YL, Zhang YJ, Radojičić A, Ding YL, Tian HN, Huang XC, Lan JM, Chen SY, Orduna AR, Zhang KW, Jetter R, Li X, Zhang YL (2020). Diverse roles of the salicylic acid receptors NPR1 and NPR3/ NPR4 in plant immunity. Plant Cell 32, 4002-4016.
DOI URL |
| [30] |
Lucas WJ, Groover A, Lichtenberger R, Furuta K, Yadav SR, Helariutta Y, He XQ, Fukuda H, Kang JL, Brady SM, Patrick JW, Sperry J, Yoshida A, López-Millán AF, Grusak MA, Kachroo P (2013). The plant vascular system: evolution, development and functions. J Integr Plant Biol 55, 294-388.
DOI URL |
| [31] |
Luna E, Bruce TJA, Roberts MR, Flors V, Ton J (2012). Next-generation systemic acquired resistance. Plant Physiol 158, 844-853.
DOI URL |
| [32] |
Memelink J (2009). Regulation of gene expression by jasmonate hormones. Phytochemistry 70, 1560-1570.
DOI PMID |
| [33] |
Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B (1990). Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250, 1004-1006.
PMID |
| [34] |
Mishina TE, Zeier J (2006). The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol 141, 1666-1675.
DOI URL |
| [35] |
Mohnike L, Rekhter D, Huang WJ, Feussner K, Tian HN, Herrfurth C, Zhang YL, Feussner I (2021). The glycosyltransferase UGT76B1 modulates N-hydroxy-pipecolic acid homeostasis and plant immunity. Plant Cell 33, 735-749.
DOI URL |
| [36] |
Mou ZL, Fan WH, Dong XN (2003). Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 113, 935-944.
DOI URL |
| [37] |
Návarová H, Bernsdorff F, Döring AC, Zeier J (2012). Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24, 5123-5141.
DOI URL |
| [38] |
Nawrath C, Métraux JP (1999). Salicylic acid induction- deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11, 1393-1404.
PMID |
| [39] |
Pálfi G, Dézsi L (1968). Pipecolic acid as an indicator of abnormal protein metabolism in diseased plants. Plant Soil 29, 285-291.
DOI URL |
| [40] |
Pallas JA, Paiva NL, Lamb C, Dixon RA (1996). Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J 10, 281-293.
DOI URL |
| [41] |
Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113-116.
DOI URL |
| [42] |
Plecko B, Hikel C, Korenke GC, Schmitt B, Baumgartner M, Baumeister F, Jakobs C, Struys E, Erwa W, Stöckler-Ipsiroglu S (2005). Pipecolic acid as a diagnostic marker of pyridoxine-dependent epilepsy. Neuropediatrics 36, 200-205.
PMID |
| [43] |
Schnake A, Hartmann M, Schreiber S, Malik J, Brahmann L, Yildiz I, von Dahlen J, Rose LE, Schaffrath U, Zeier J (2020). Inducible biosynthesis and immune function of the systemic acquired resistance inducer N-hydroxypipecolic acid in monocotyledonous and dicotyledonous plants. J Exp Bot 71, 6444-6459.
DOI PMID |
| [44] | Shah J, Zeier J (2013). Long-distance communication and signal amplification in systemic acquired resistance. Front Plant Sci 4, 30. |
| [45] |
Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch- Mani B (2012). Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol 158, 835-843.
DOI PMID |
| [46] |
Song JT, Lu H, Greenberg JT (2004a). Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, ABERRANT GROWTH AND DEATH 2 and AGD2-LIKE DEFENSE RESPONSE PROTEIN 1, encoding novel aminotransferases. Plant Cell 16, 353-366.
DOI URL |
| [47] |
Song JT, Lu H, McDowell JM, Greenberg JT (2004b). A key role for ALD1 in activation of local and systemic defenses in Arabidopsis. Plant J 40, 200-212.
DOI URL |
| [48] |
Sun TJ, Huang JH, Xu Y, Verma V, Jing BB, Sun YL, Ruiz Orduna A, Tian HN, Huang XC, Xia ST, Schafer L, Jetter R, Zhang YL, Li X (2020). Redundant CAMTA transcription factors negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by modulating the expression of SARD1 and CBP60g. Mol Plant 13, 144-156.
DOI URL |
| [49] |
Sun TJ, Zhang YX, Li Y, Zhang Q, Ding YL, Zhang YL (2015). ChIP-seq reveals broad roles of SARD1 and CBP60g in regulating plant immunity. Nat Commun 6, 10159.
DOI URL |
| [50] |
Thibaud-Nissen F, Wu H, Richmond T, Redman JC, Johnson C, Green R, Arias J, Town CD (2006). Development of Arabidopsis whole-genome microarrays and their application to the discovery of binding sites for the TGA2 transcription factor in salicylic acid-treated plants. Plant J 47, 152-162.
PMID |
| [51] |
Tian HN, Zhang YL (2019). The emergence of a mobile signal for systemic acquired resistance. Plant Cell 31, 1414-1415.
DOI URL |
| [52] |
Tranchant C, Aubourg P, Mohr M, Rocchiccioli F, Zaenker C, Warter JM (1993). A new peroxisomal disease with impaired phytanic and pipecolic acid oxidation. Neurology 43, 2044-2048.
PMID |
| [53] |
Trapp S, Croteau R (2001). Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52, 689-724.
DOI URL |
| [54] |
van Loon LC, Rep M, Pieterse CMJ (2006). Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44, 135-162.
PMID |
| [55] |
Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J (1994). Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6, 959-965.
DOI URL |
| [56] |
Vicente J, Cascón T, Vicedo B, García-Agustín P, Hamberg M, Castresana C (2012). Role of 9-lipoxygenase and α-dioxygenase oxylipin pathways as modulators of local and systemic defense. Mol Plant 5, 914-928.
DOI PMID |
| [57] |
Vogel-Adghough D, Stahl E, Návarová H, Zeier J (2013). Pipecolic acid enhances resistance to bacterial infection and primes salicylic acid and nicotine accumulation in tobacco. Plant Signal Behav 8, e26366.
DOI URL |
| [58] | Wang CX, Liu RY, Lim GH, de Lorenzo L, Yu KS, Zhang K, Hunt AG, Kachroo A, Kachroo P (2018a). Pipecolic acid confers systemic immunity by regulating free radicals. Sci Adv 4, eaar4509. |
| [59] |
Wang YM, Schuck S, Wu JN, Yang P, Döring AC, Zeier J, Tsuda K (2018b). A MPK3/6-WRKY33-ALD1-pipecolic acid regulatory loop contributes to systemic acquired resistance. Plant Cell 30, 2480-2494.
DOI URL |
| [60] |
Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Després C (2012). The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1, 639-647.
DOI URL |
| [61] |
Yalpani N, Silverman P, Wilson TM, Kleier DA, Raskin I (1991). Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell 3, 809-818.
PMID |
| [62] |
Yildiz I, Mantz M, Hartmann M, Zeier T, Kessel J, Thurow C, Gatz C, Petzsch P, Köhrer K, Zeier J (2021). The mobile SAR signal N-hydroxypipecolic acid induces NPR1- dependent transcriptional reprogramming and immune priming. Plant Physiol 186, 1679-1705.
DOI PMID |
| [63] |
Zeier J (2013). New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ 36, 2085-2103.
DOI URL |
| [64] |
Zoeller M, Stingl N, Krischke M, Fekete A, Waller F, Berger S, Mueller MJ (2012). Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiol 160, 365-378.
DOI PMID |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||