

Chinese Bulletin of Botany ›› 2015, Vol. 50 ›› Issue (1): 90-99.DOI: 10.3724/SP.J.1259.2015.00090 cstr: 32102.14.SP.J.1259.2015.00090
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Chuanyuan Deng1, *, Guiliang Xin1, Wanchao Zhang1, Suzhi Guo2, Qiuhua Xue1, Zhongxiong Lai3, Luying Ye1
Received:2013-12-16
Accepted:2014-11-13
Online:2015-01-01
Published:2015-04-09
Contact:
Deng Chuanyuan
About author:? These authors contributed equally to this paper
Chuanyuan Deng, Guiliang Xin, Wanchao Zhang, Suzhi Guo, Qiuhua Xue, Zhongxiong Lai, Luying Ye. SEM Observations and Measurements of Vestured Pits of the Secondary Xylem in the Tribe Rhizophoreae[J]. Chinese Bulletin of Botany, 2015, 50(1): 90-99.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.chinbullbotany.com/EN/10.3724/SP.J.1259.2015.00090
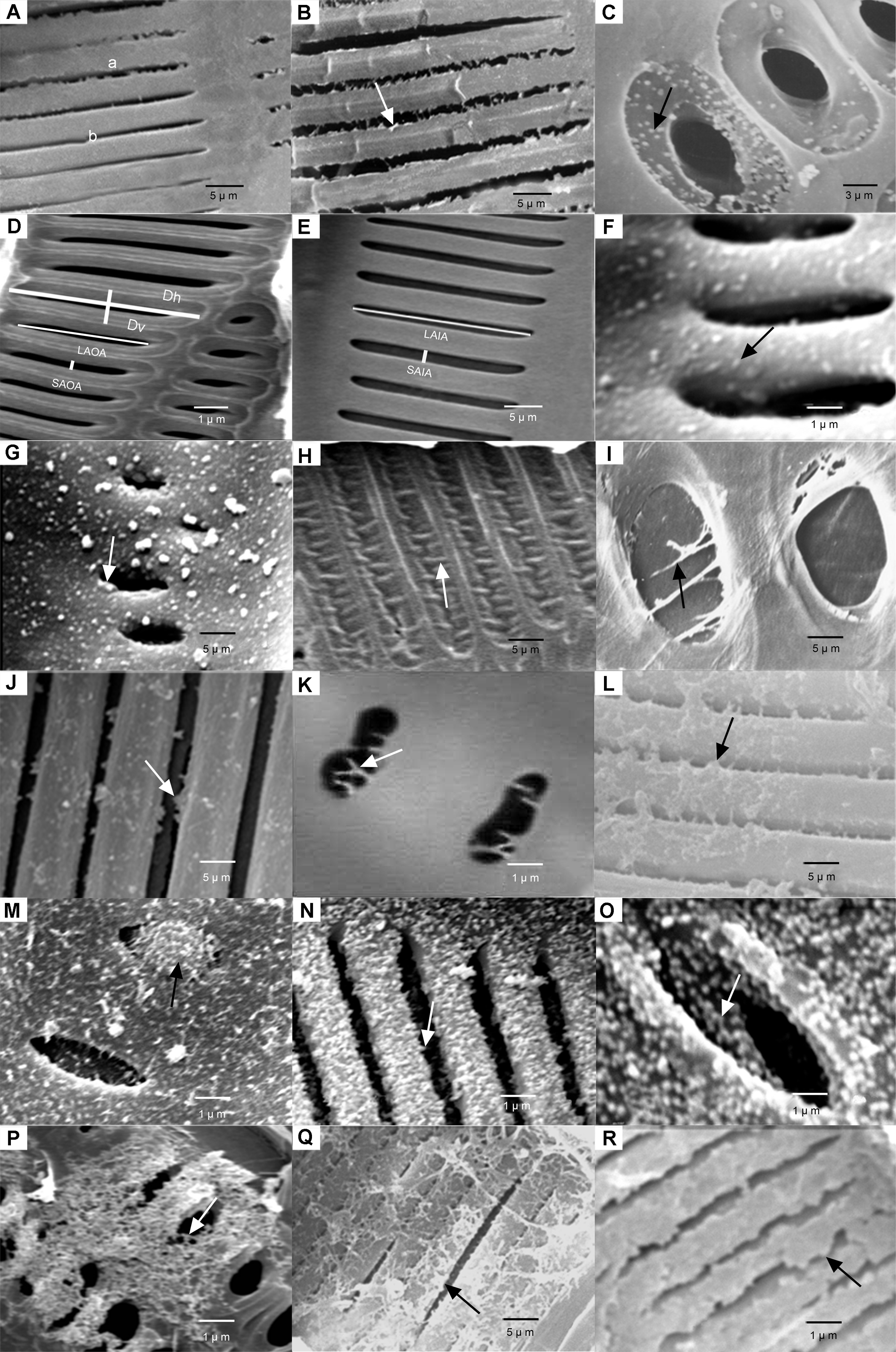
Figure 1 Distribution and micromorphology of vestured pits in the study species of tribe Rhizophoreae (A) Ceriops tagal, intervascular pits in the wall of a vessel element viewed from the lumen side, the inner pit apertures are vestured (a), or non-vestured (b); (B) Kandelia candel, intervascular pits in the wall of a vessel element viewed from the outer surface, arrow shows the outer pit apertures are vestured; (C) Rhizophora stylosa, vessel-ray parenchyma pits in the wall of a vessel element viewed from the outer surface, arrow shows the pit chambers are vestured; (D) Rhizophora mucronata, non-vestured outer pit apertures and non-vestured pit chambers in the wall of a vessel element viewed from the outer surface (Dh: Horizontal pit membrane diameter; Dv: Vertical pit membrane diameter; LAOA: The longest axis of the outer aperture; SAOA: The shortest axis of the outer aperture); (E) Ceriops australis, non-vestured inner pit apertures in the wall of a vessel element viewed from the lumen side (LAIA: The longest axis of the inner aperture; SAIA: The shortest axis of the inner aperture); (F) Rhizophora stylosa, intervascular pits in the wall of a vessel element viewed from lumen side, arrow shows scattered dot-shaped vestures occur in the inner pit apertures; (G) Rhizophora stylosa, vessel-ray parenchyma pits in the wall of a vessel element viewed from the lumen side, arrow shows scattered dot-shaped vestures occur in the inner pit apertures; (H) Rhizophora mangle, intervascular pits in the wall of a vessel element viewed from the outer surface, arrow shows scattered rod-shaped vestures occur in the pit chambers; (I) Bruguiera sexangula var. rhynchopetala, vessel-ray parenchyma pits in the wall of a vessel element viewed from the outer surface, arrow shows scattered silk-like vestures occur in the outer pit apertures; (J) Bruguiera gymnorrhiza, intervascular pits in the wall of a vessel element viewed from the outer surface, arrow shows forked vestures occur in the outer pit apertures; (K) Bruguiera gymnorrhiza, vessel-ray parenchyma pits in the wall of a vessel element viewed from the lumen side, arrow shows forked vestures occur in the inner pit apertures; (L) Rhizophora apiculata, intervascular pits in the wall of a vessel element viewed from the lumen side, arrow shows forked vestures together with dot-like vestures arise from the margin of the inner pit aperture, therefore, the irregular shape of inner pit aperture develop; (M) Bruguiera gymnorrhiza, vessel-ray parenchyma pits in the wall of a vessel element viewed from the lumen side, arrow shows dot-like and rod-like vestures aggregate into a agglomerate and occlude the inner pit aperture; (N) Kandelia candel, intervascular pits in the wall of a vessel element viewed from the outer surface, arrow shows aggregate dot-shaped or rod-shaped vestures occur in the outer pit apertures and pit chambers; (O) Bruguiera gymnorrhiza, vessel-ray parenchyma pits in the wall of a vessel element viewed from the outer surface, arrow shows aggregate dot-shaped or rod-shaped vestures occur in the outer pit apertures and pit chambers; (P) Bruguiera sexangula, vessel-ray parenchyma pits in the wall of a vessel element viewed from the outer surface, arrow shows filament-like vestures in the outer pit apertures or pit chambers interweave to form into a rather compact network and extend to the outside of pits; (Q) Ceriops tagal, intervascular pits in the wall of a vessel element viewed from the lumen side, arrow shows filament-like vestures in the inner pit apertures interweave to form into a network and extend to the inner vessel wall; (R) Ceriops australis, arrow shows sheeted vestures occurred in the wall of a vessel element viewed from the lumen side and obscure the inner pit apertures
| Scientific name | Inner pit aperture | Outer pit aperture | Pit chamber | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |||||
| Bruguiera gyrmnorrhiza | + | + | + | ++ | ++ | ++ | + | + | + | – | ++ | ++ | + | ++ | + | + | - | - | ++ | + | + | ||||
| B. parviflora | + | + | - | + | - | - | + | + | + | - | + | - | - | + | + | - | - | - | - | + | |||||
| B. sexangula | + | + | + | ++ | ++ | + | + | + | + | - | + | - | + | ++ | + | + | - | - | - | + | + | ||||
| var. rhynchuopetala | ++ | + | - | + | + | - | - | + | ++ | + | + | - | - | - | + | + | - | - | - | - | - | ||||
| Ceriops australis | + | + | + | ++ | ++ | + | ++ | + | + | + | + | - | - | - | ++ | + | - | - | - | - | - | ||||
| C. tagal | ++ | + | + | ++ | ++ | ++ | + | ++ | + | - | ++ | - | + | + | + | ++ | - | + | - | - | + | ||||
| Kandelia candel | ++ | + | + | ++ | ++ | ++ | + | + | + | + | ++ | + | - | ++ | + | + | - | - | + | + | + | ||||
| Rhizophora apiculata | + | + | + | ++ | ++ | + | ++ | + | + | - | + | - | - | + | + | - | - | - | + | - | - | ||||
| R. mangle | + | + | + | ++ | ++ | + | ++ | + | + | + | + | - | - | ++ | + | + | + | - | - | - | + | ||||
| R. mucronata | + | + | + | + | ++ | + | + | + | + | - | + | + | - | + | + | ++ | + | - | + | - | - | ||||
| R. stylosa | ++ | + | + | + | ++ | ++ | + | + | + | + | + | + | - | + | + | + | - | + | + | + | + | ||||
Table 1 Distribution and micromorphological variations of vestures in pits of Rhizophoreae species studied
| Scientific name | Inner pit aperture | Outer pit aperture | Pit chamber | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |||||
| Bruguiera gyrmnorrhiza | + | + | + | ++ | ++ | ++ | + | + | + | – | ++ | ++ | + | ++ | + | + | - | - | ++ | + | + | ||||
| B. parviflora | + | + | - | + | - | - | + | + | + | - | + | - | - | + | + | - | - | - | - | + | |||||
| B. sexangula | + | + | + | ++ | ++ | + | + | + | + | - | + | - | + | ++ | + | + | - | - | - | + | + | ||||
| var. rhynchuopetala | ++ | + | - | + | + | - | - | + | ++ | + | + | - | - | - | + | + | - | - | - | - | - | ||||
| Ceriops australis | + | + | + | ++ | ++ | + | ++ | + | + | + | + | - | - | - | ++ | + | - | - | - | - | - | ||||
| C. tagal | ++ | + | + | ++ | ++ | ++ | + | ++ | + | - | ++ | - | + | + | + | ++ | - | + | - | - | + | ||||
| Kandelia candel | ++ | + | + | ++ | ++ | ++ | + | + | + | + | ++ | + | - | ++ | + | + | - | - | + | + | + | ||||
| Rhizophora apiculata | + | + | + | ++ | ++ | + | ++ | + | + | - | + | - | - | + | + | - | - | - | + | - | - | ||||
| R. mangle | + | + | + | ++ | ++ | + | ++ | + | + | + | + | - | - | ++ | + | + | + | - | - | - | + | ||||
| R. mucronata | + | + | + | + | ++ | + | + | + | + | - | + | + | - | + | + | ++ | + | - | + | - | - | ||||
| R. stylosa | ++ | + | + | + | ++ | ++ | + | + | + | + | + | + | - | + | + | + | - | + | + | + | + | ||||
| Species | FVIA (%) | FVOA (%) | FVPC (%) | Dh (μm) | Dv (μm) | LAOA (μm) | SAOA (μm) | LAIA (μm) | SAIA (μm) | APf | Fap (%) | PA (μm2) | OPA (μm2) | IPA (μm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruguiera gymnorrhiza | 27.77± 44.57 | 16.40± 36.07 | 14.53± 34.25 | 23.47± 7.26 | 4.72± 1.90 | 12.56± 7.27 | 1.98± 1.05 | 18.62± 7.20 | 1.43± 0.62 | 7.56± 6.05 | 21.10± 9.16 | 95.68± 67.45 | 21.31± 19.68 | 20.94± 12.36 |
| B. parviflora | 19.09± 34.63 | 7.78± 23.00 | 5.75± 21.40 | 23.02± 7.50 | 4.57± 1.03 | 14.65± 5.97 | 1.02± 0.78 | 14.45± 5.62 | 1.02± 0.58 | 18.83± 11.66 | 14.74± 11.90 | 87.60± 44.24 | 13.02± 13.73 | 11.58± 10.60 |
| B. sexangula | 26.25± 39.06 | 15.06± 37.89 | 6.76± 19.60 | 27.47± 10.40 | 3.72± 1.21 | 21.72± 8.78 | 1.13± 0.90 | 17.94± 5.02 | 1.44± 0.95 | 24.91± 12.76 | 23.12± 12.37 | 83.49± 48.62 | 20.83± 18.34 | 20.99± 18.61 |
| B. sexangula var. rhynchuopetala | 20.54± 40.21 | 11.21± 39.39 | 4.21± 14.24 | 23.51± 9.00 | 3.70± 1.12 | 17.96± 7.53 | 0.78± 0.30 | 12.93± 4.34 | 1.28± 0.63 | 25.55± 12.11 | 16.06± 5.71 | 74.13± 52.31 | 12.11± 9.44 | 13.21± 8.34 |
| Ceriops australis | 21.82± 35.69 | 7.04± 21.32 | 8.83± 24.26 | 25.00± 9.14 | 3.46± 1.05 | 20.25± 8.07 | 0.79± 0.48 | 15.14± 5.58 | 0.71± 0.44 | 32.74± 17.60 | 18.23± 11.00 | 71.26± 40.25 | 12.82± 9.06 | 8.94± 7.87 |
| C. tagal | 25.01± 40.81 | 1.53± 7.97 | 0.48± 3.09 | 15.00± 3.78 | 2.68± 0.99 | 12.29± 3.63 | 0.59± 0.21 | 18.44± 7.58 | 1.31± 1.10 | 24.16± 13.64 | 21.84± 15.01 | 19.89± 20.22 | 18.10± 14.75 | 21.75± 25.90 |
| Kandelia candel | 31.56± 39.40 | 5.82± 18.16 | 1.04± 8.14 | 24.76± 7.75 | 4.98± 1.64 | 18.90± 5.72 | 1.42± 0.59 | 20.91± 7.75 | 1.33± 0.56 | 15.28± 6.53 | 22.13± 7.52 | 105.31± 71.20 | 22.89± 15.84 | 24.10± 18.28 |
| Rhizophora apiculata | 25.94± 35.87 | 8.83± 22.65 | 1.71± 7.14 | 29.84± 10.74 | 4.80± 1.49 | 21.30± 9.92 | 1.28± 1.05 | 27.98± 11.78 | 1.67± 0.71 | 21.62± 14.31 | 17.86± 9.33 | 117.43± 68.31 | 24.25± 34.96 | 35.69± 20.76 |
| R. mangle | 25.97± 37.30 | 5.82± 19.78 | 2.17± 13.38 | 18.03± 5.08 | 3.33± 0.61 | 13.82± 4.23 | 1.03± 0.46 | 13.32± 4.04 | 1.23± 1.19 | 16.04± 7.71 | 13.57± 9.76 | 189.41± 63.61 | 41.48± 13.45 | 13.14± 15.49 |
| R. mucronata | 26.93± 42.66 | 9.40± 25.61 | 6.22± 20.95 | 26.47± 5.77 | 4.11± 1.17 | 19.52± 4.08 | 1.03± 0.44 | 19.34± 4.77 | 1.42± 0.50 | 22.80± 12.01 | 19.24± 10.79 | 88.04± 42.51 | 16.09± 9.57 | 21.92± 9.58 |
| R. stylosa | 34.43± 45.00 | 26.97± 42.95 | 15.79± 37.46 | 18.89± 6.36 | 2.18± 1.32 | 11.99± 5.77 | 1.17± 0.59 | 18.70± 2.34 | 1.78± 0.32 | 14.47± 12.97 | 41.60± 19.29 | 33.10± 31.83 | 9.50± 3.39 | 26.34± 5.91 |
| PI | 0.45 | 0.94 | 0.97 | 0.50 | 0.56 | 0.45 | 0.70 | 0.54 | 0.60 | 0.77 | 0.67 | 0.89 | 0.77 | 0.75 |
Table 2 Richness indexes of intervascular vestured pits and quantitative features of scalariform intervascular bordered pits in 10 species and 1 variety of tribe Rhizophoreae (means±SD)
| Species | FVIA (%) | FVOA (%) | FVPC (%) | Dh (μm) | Dv (μm) | LAOA (μm) | SAOA (μm) | LAIA (μm) | SAIA (μm) | APf | Fap (%) | PA (μm2) | OPA (μm2) | IPA (μm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruguiera gymnorrhiza | 27.77± 44.57 | 16.40± 36.07 | 14.53± 34.25 | 23.47± 7.26 | 4.72± 1.90 | 12.56± 7.27 | 1.98± 1.05 | 18.62± 7.20 | 1.43± 0.62 | 7.56± 6.05 | 21.10± 9.16 | 95.68± 67.45 | 21.31± 19.68 | 20.94± 12.36 |
| B. parviflora | 19.09± 34.63 | 7.78± 23.00 | 5.75± 21.40 | 23.02± 7.50 | 4.57± 1.03 | 14.65± 5.97 | 1.02± 0.78 | 14.45± 5.62 | 1.02± 0.58 | 18.83± 11.66 | 14.74± 11.90 | 87.60± 44.24 | 13.02± 13.73 | 11.58± 10.60 |
| B. sexangula | 26.25± 39.06 | 15.06± 37.89 | 6.76± 19.60 | 27.47± 10.40 | 3.72± 1.21 | 21.72± 8.78 | 1.13± 0.90 | 17.94± 5.02 | 1.44± 0.95 | 24.91± 12.76 | 23.12± 12.37 | 83.49± 48.62 | 20.83± 18.34 | 20.99± 18.61 |
| B. sexangula var. rhynchuopetala | 20.54± 40.21 | 11.21± 39.39 | 4.21± 14.24 | 23.51± 9.00 | 3.70± 1.12 | 17.96± 7.53 | 0.78± 0.30 | 12.93± 4.34 | 1.28± 0.63 | 25.55± 12.11 | 16.06± 5.71 | 74.13± 52.31 | 12.11± 9.44 | 13.21± 8.34 |
| Ceriops australis | 21.82± 35.69 | 7.04± 21.32 | 8.83± 24.26 | 25.00± 9.14 | 3.46± 1.05 | 20.25± 8.07 | 0.79± 0.48 | 15.14± 5.58 | 0.71± 0.44 | 32.74± 17.60 | 18.23± 11.00 | 71.26± 40.25 | 12.82± 9.06 | 8.94± 7.87 |
| C. tagal | 25.01± 40.81 | 1.53± 7.97 | 0.48± 3.09 | 15.00± 3.78 | 2.68± 0.99 | 12.29± 3.63 | 0.59± 0.21 | 18.44± 7.58 | 1.31± 1.10 | 24.16± 13.64 | 21.84± 15.01 | 19.89± 20.22 | 18.10± 14.75 | 21.75± 25.90 |
| Kandelia candel | 31.56± 39.40 | 5.82± 18.16 | 1.04± 8.14 | 24.76± 7.75 | 4.98± 1.64 | 18.90± 5.72 | 1.42± 0.59 | 20.91± 7.75 | 1.33± 0.56 | 15.28± 6.53 | 22.13± 7.52 | 105.31± 71.20 | 22.89± 15.84 | 24.10± 18.28 |
| Rhizophora apiculata | 25.94± 35.87 | 8.83± 22.65 | 1.71± 7.14 | 29.84± 10.74 | 4.80± 1.49 | 21.30± 9.92 | 1.28± 1.05 | 27.98± 11.78 | 1.67± 0.71 | 21.62± 14.31 | 17.86± 9.33 | 117.43± 68.31 | 24.25± 34.96 | 35.69± 20.76 |
| R. mangle | 25.97± 37.30 | 5.82± 19.78 | 2.17± 13.38 | 18.03± 5.08 | 3.33± 0.61 | 13.82± 4.23 | 1.03± 0.46 | 13.32± 4.04 | 1.23± 1.19 | 16.04± 7.71 | 13.57± 9.76 | 189.41± 63.61 | 41.48± 13.45 | 13.14± 15.49 |
| R. mucronata | 26.93± 42.66 | 9.40± 25.61 | 6.22± 20.95 | 26.47± 5.77 | 4.11± 1.17 | 19.52± 4.08 | 1.03± 0.44 | 19.34± 4.77 | 1.42± 0.50 | 22.80± 12.01 | 19.24± 10.79 | 88.04± 42.51 | 16.09± 9.57 | 21.92± 9.58 |
| R. stylosa | 34.43± 45.00 | 26.97± 42.95 | 15.79± 37.46 | 18.89± 6.36 | 2.18± 1.32 | 11.99± 5.77 | 1.17± 0.59 | 18.70± 2.34 | 1.78± 0.32 | 14.47± 12.97 | 41.60± 19.29 | 33.10± 31.83 | 9.50± 3.39 | 26.34± 5.91 |
| PI | 0.45 | 0.94 | 0.97 | 0.50 | 0.56 | 0.45 | 0.70 | 0.54 | 0.60 | 0.77 | 0.67 | 0.89 | 0.77 | 0.75 |
| 1 | 邓传远, 郭素枝, 林鹏 (2004a). 海桑属(Sonneratia)植物的木材结构及其系统演化意义. 热带亚热带植物学报 12, 213-220. |
| 2 | 邓传远, 林鹏, 郭素枝 (2004b). 海桑属红树植物次生木质部解剖特征及其对潮间带生境的适应. 植物生态学报 28, 392-399. |
| 3 | 邓传远, 林鹏, 郭素枝 (2004c). 榄李属(Lumnitzera)红树植物的木材解剖学研究. 厦门大学学报(自然科学版) 43, 406-411. |
| 4 | Ashton PMS, Olander LP, Berlyn GP, Thadani R, Cameron IR (1998). Changes in leaf structure in relation to crown position and tree size of Betula papyrifera within fire-origin stands of interior cedar-hemlock.Can J Bot 76, 1180-1187. |
| 5 | Baas P, Wheeler E, Chase M (2000). Dicotyledonous wood anatomy and the APG system of angiosperm classification.Bot J Linnean Soc 134, 3-17. |
| 6 | Carlquist S (2001). Comparative Wood Anatomy, 2nd edn. Berlin: Springer Verlag. |
| 7 | Carlquist S (2012). How wood evolves: a new synthesis.Botany 90, 901-940. |
| 8 | Choat B, Brodie TW, Cobb AR, Zwieniecki MA, Holbrook NM (2006). Direct measurement of intervessel pit membrance hydraulic resistance in two angiosperm tree species.Am J Bot 93, 993-1000. |
| 9 | Choat B, Jansen S, Zwieniecki MA, Smets E, Holbrook NM (2004). Changes in pit membrane porosity due to deflection and stretching: the role of vestured pits.J Exp Bot 55, 1569-1575. |
| 10 | IAWA Committee (1989). IAWA list of microscopic features for hardwood identification.IAWA Bulletin ns 10, 219-332. |
| 11 | Jansen S, Baas P, Gasson P, Lens F, Smets E (2004). Variation in xylem structure from tropics to tundra: evidence from vestured pits.Proc Natl Acad Sci USA 101, 8833-8837. |
| 12 | Jansen S, Baas P, Gasson P, Smets E (2003). Vestured pits: do they promote safer water transport?Int J Plant Sci 164, 405-413. |
| 13 | Jansen S, Baas P, Smets E (2001). Vestured pits: their occurrence and systematic importance in eudicots.Taxon 50, 135-167. |
| 14 | Jansen S, Kitin P, De Pauw H, Idris M, Beeckman H, Smets E (1998a). Preparation of wood specimens for transmitted light microscopy and scanning electron microscopy.Belg J Bot 131, 41-49. |
| 15 | Jansen S, Piesschaert F, Smets E (2000). Wood anatomy of Elaeagnaceae, with comments on vestured pits, helical thickenings, and systematic relationships.Am J Bot 87, 20-28. |
| 16 | Jansen S, Pletsers A, Rabaey D, Lens F (2008). Vestured pits: a diagnostic character in the secondary xylem of Myrtales.J Trop For Sci 20, 328-339. |
| 17 | Jansen S, Robbrecht E, Beeckman H, Smets E (2002). A survey of the systematic wood anatomy of the Rubiaceae.IAWA J 23, 1-67. |
| 18 | Jansen S, Smets E, Baas P (1998b). Vestures in woody plants: a review.IAWA J 19, 347-382. |
| 19 | Kohonen MM, Helland A (2009). On the function of wall sculpturing in xylem conduits.J Bionics Eng 6, 324-329. |
| 20 | Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S (2011). Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer.New Phytol 190, 709-723. |
| 21 | Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S (2013). Embolism resistance as a key mechanism to understand adaptive plant strategies.Curr Opin Plant Biol 16, 287-292. |
| 22 | Meylan BA, Butterfield BG (1974). Occurrence of vestured pits in the vessels and fibres of New Zealand woods.NZ J Bot 12, 3-18. |
| 23 | Ohtani J, Ishida S (1976). Study on the pit of wood cells using scanning electron microscopy. Report 5. Vestured pits in Japanese dicotyledonous woods.Res Bull CoIl Exp For Hokkaido Univ 33, 407-435. |
| 24 | Rabaey D, Lens F, Smets E, Jansen S (2010). The phylogenetic significance of vestured pits in Boraginaceae.Taxon 59, 510-516. |
| 25 | Schmitz N, Jansen S, Verheyden A, Kairo JG, Beeckman H, Koedam N (2007). Comparative anatomy of intervessel pits in two mangrove species growing along a natural salin- ity gradient in Gazi Bay, Kenya.Ann Bot 100, 271-281. |
| 26 | Schmitz N, Koch G, Schmitt U, Beeckman H, Koedam N (2008). Intervessel pit structure and histochemistry of two mangrove species as revealed by cellular UV microspectrophotometry and electron microscopy: intraspecific variation and functional significance.Microsc Microanal 14, 387-397. |
| 27 | Schwarzbach AE, Ricklefs RE (2000). Systematic affinities of Rhizophoraceae and Anisophylleaceae, and intergeneric relationships within Rhizophoraceae, based on chloroplast DNA, nuclear ribosomal DNA, and morpho- logy.Am J Bot 87, 547-564. |
| 28 | Van Vliet GJCM (1976). Wood anatomy of the Rhizophoraceae.Leiden Bot Ser 3, 20-75. |
| 29 | Wheeler EA, Baas P, Rodgers S (2007). Variations in dieot wood anatomy: a global analysis based on the Inside Wood database.IAWA J 28, 229-258. |
| 30 | Wu J, Ohtani J, Fukazawa K (1989). SEM observations on the vessel wall modifications in Yunnan hardwoods.Res Bull CoIl Exp For Hokkaido Univ 46, 847-939. |
| 31 | Wurdack KJ, Davis CC (2009). Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life.Am J Bot 96, 1551-1570. |
| 32 | Zweypfenning RCVJ (1978). A hypothesis on the function of vestured pits.IAWA Bull 1, 13-15. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||