

Chinese Bulletin of Botany ›› 2016, Vol. 51 ›› Issue (5): 667-678.DOI: 10.11983/CBB15190 cstr: 32102.14.CBB15190
Previous Articles Next Articles
Nianwei Qiu1†, Xiushun Wang1†, Fabin Yang1, Xiaogang Yang1, Wen Yang1, Runjie Diao1, Xiu Wang1, Jing Cui1, Feng Zhou2*
Received:2015-10-23
Accepted:2016-02-27
Online:2016-09-01
Published:2016-09-27
Contact:
Qiu Nianwei,Wang Xiushun,Zhou Feng
About author:# Co-first authors
Nianwei Qiu, Xiushun Wang, Fabin Yang, Xiaogang Yang, Wen Yang, Runjie Diao, Xiu Wang, Jing Cui, Feng Zhou. Fast Extraction and Precise Determination of Chlorophyll[J]. Chinese Bulletin of Botany, 2016, 51(5): 667-678.
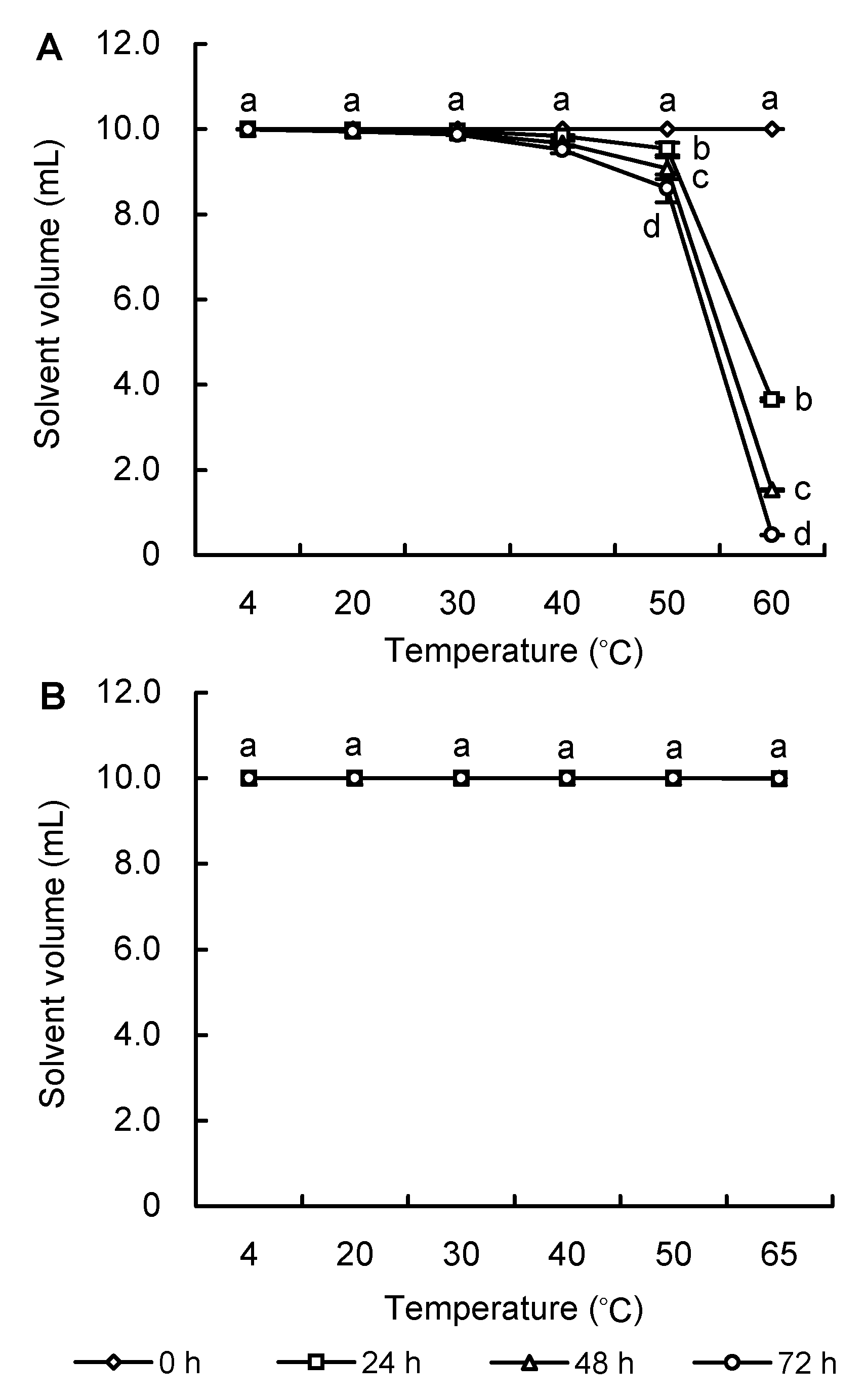
Figure 1 The volatility of 80% acetone (A) and DMSO (B) under different temperatures Different lowercase letters indicated significant difference at P<0.05.
| Plant material | 80% acetone | DMSO | |||||
|---|---|---|---|---|---|---|---|
| 4°C | 20°C | 30°C | 40°C | 50°C | 65°C | ||
| Spinach leaf | 79.3±6.7 a | 71.2±5.4 b | 47.6±3.6 c | 38.7±4.2 d | 25.6±3.7 e | 1.08±0.04 f | |
| Wheat leaf | 28.7±3.1 a | 23.1±2.3 b | 17.9±1.8 c | 14.8±1.2 d | 8.3±0.7 e | 0.82±0.07 f | |
| Chinese pine leaf | 66.7±4.8 a | 42.4±3.4 b | 27.3±2.5 c | 23.6±2.1 d | 17.5±1.6 e | 2.08±0.14 f | |
Table 1 The extraction time of immersion extraction with DMSO and 80% acetone under different temperatures (unit: h)
| Plant material | 80% acetone | DMSO | |||||
|---|---|---|---|---|---|---|---|
| 4°C | 20°C | 30°C | 40°C | 50°C | 65°C | ||
| Spinach leaf | 79.3±6.7 a | 71.2±5.4 b | 47.6±3.6 c | 38.7±4.2 d | 25.6±3.7 e | 1.08±0.04 f | |
| Wheat leaf | 28.7±3.1 a | 23.1±2.3 b | 17.9±1.8 c | 14.8±1.2 d | 8.3±0.7 e | 0.82±0.07 f | |
| Chinese pine leaf | 66.7±4.8 a | 42.4±3.4 b | 27.3±2.5 c | 23.6±2.1 d | 17.5±1.6 e | 2.08±0.14 f | |
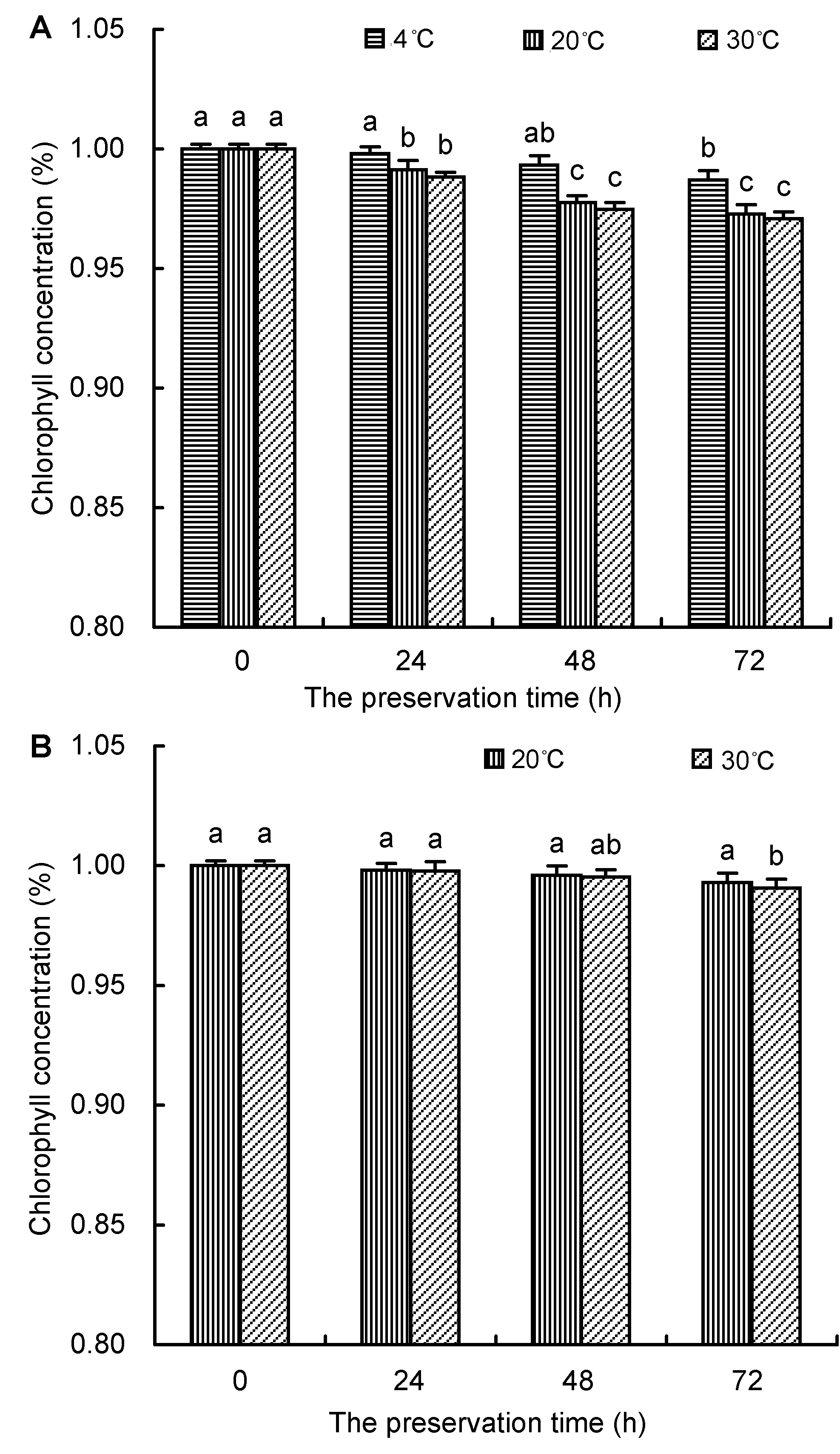
Figure 3 The stability of chlorophyll in 80% acetone (A) and DMSO (B) under different temperatures Different lowercase letters indicated significant difference at P<0.05.
| DMSO:80% acetone (v/v) | |||
|---|---|---|---|
| 0:5 | 1:4 | 1:1 | |
| Wavelength of Chla (nm) | 663.7±0.03 (663.3-664.1) | 663.2±0.03 (663.0-663.4) | 663.9±0.05 (663.6-664.2) |
| Wavelength of Chlb (nm) | 647.0±0.04 (646.6-647.4) | 646.8±0.02 (646.4-647.2) | 647.8±0.04 (647.5-651.1) |
Table 2 The absorption peak (nm) of chlorophyll in miscible liquids of DMSO and 80% acetone (means±SD)
| DMSO:80% acetone (v/v) | |||
|---|---|---|---|
| 0:5 | 1:4 | 1:1 | |
| Wavelength of Chla (nm) | 663.7±0.03 (663.3-664.1) | 663.2±0.03 (663.0-663.4) | 663.9±0.05 (663.6-664.2) |
| Wavelength of Chlb (nm) | 647.0±0.04 (646.6-647.4) | 646.8±0.02 (646.4-647.2) | 647.8±0.04 (647.5-651.1) |
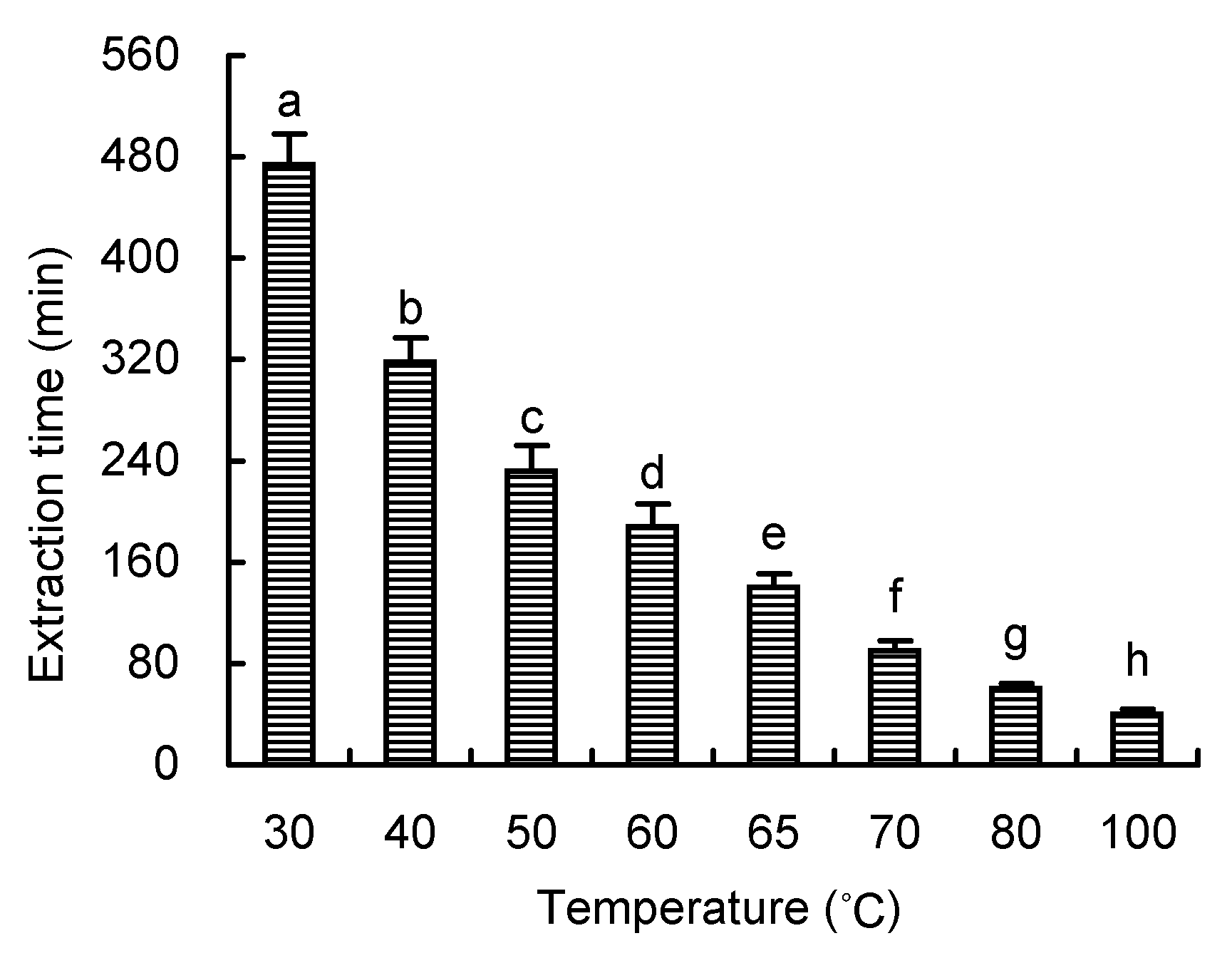
Figure 5 The time of extracting chlorophyll from spinach leaf with DMSO under different temperatures Different lowercase letters indicated significant difference at P<0.05.
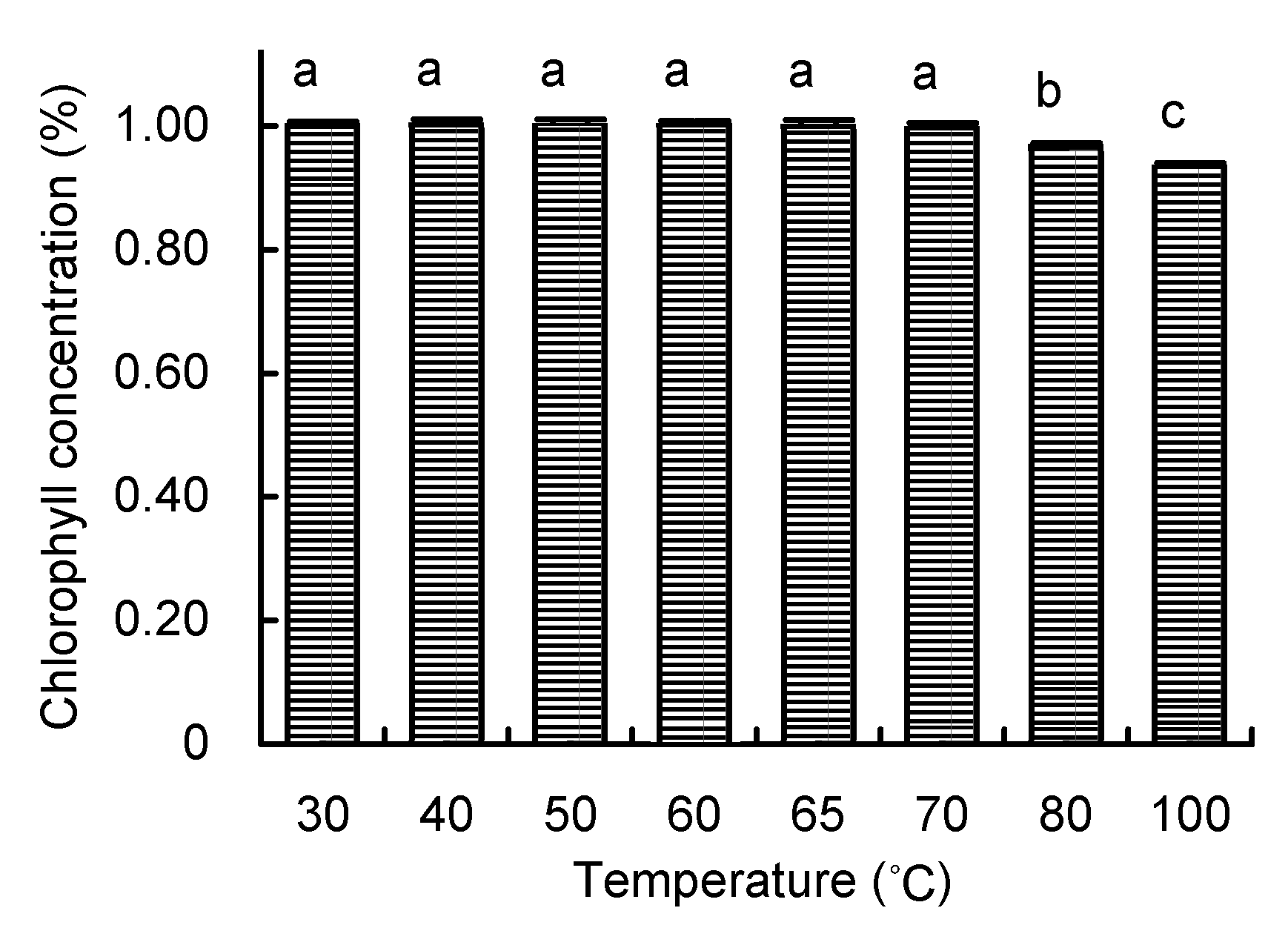
Figure 6 The degradation of chlorophyll in DMSO solution under different temperatures Different lowercase letters indicated significant difference at P<0.05.
| Chla solution | Chlb solution | ||||||
|---|---|---|---|---|---|---|---|
| 663.2 nm | 663.6 nm | 663.7 nm | 646.6 nm | 646.8 nm | 647.0 nm | ||
| 80% acetone | 0.852 | 0.853 | 0.853 | 0.498 | 0.498 | 0.498 | |
| 1:4 miscible solvent | 0.853 | 0.852 | 0.852 | 0.501 | 0.501 | 0.501 | |
Table 3 Comparison of chlorophyll absorbance at absorption peak wavelength in two solvents
| Chla solution | Chlb solution | ||||||
|---|---|---|---|---|---|---|---|
| 663.2 nm | 663.6 nm | 663.7 nm | 646.6 nm | 646.8 nm | 647.0 nm | ||
| 80% acetone | 0.852 | 0.853 | 0.853 | 0.498 | 0.498 | 0.498 | |
| 1:4 miscible solvent | 0.853 | 0.852 | 0.852 | 0.501 | 0.501 | 0.501 | |
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Formulas | References | ||||||
| 1 | Ca (mg·L-1)=12.7A 663-2.69A645; Cb (mg·L-1)=22.9A645-4.68A663 | Arnon, 1949 | ||||||
| 2 | Ca (mg·L-1)=12.72A663-2.59A645; Cb (mg·L-1)=22.88A645-4.67A663 | 陈毓荃, 1986 | ||||||
| 3 | Ca (mg·L-1)=12.63A664.5-2.52A647; Cb (mg·L-1)=20.47A647-4.73A664.5 | Inskeep and Bloom, 1985 | ||||||
| 4 | Ca (mg·L-1)=12.21A663-2.81A646; Cb (mg·L-1)=20.13A646-5.03A663 | Wellburn and Lichtenthaler, 1984 | ||||||
| 5 | Ca (mg·L-1)=12.25A663.2-2.79A646.8; Cb (mg·L-1)=21.50A646.8-5.10A663.2 | Lichtenthaler, 1987 | ||||||
| 6 | Ca (mg·L-1)=12.25A663.6-2.55A646.6; Cb (mg·L-1)=20.31A646.6-4.91A663.6 | Porra et al., 1989 | ||||||
| 7 | Ca (mg·L-1)=12.27A663.6-2.52A646.6; Cb (mg·L-1)=20.10A646.6-4.92A663.6 (correction formula of DMSO:80% acetone=1:4 (v/v) mixed solvent) | - | ||||||
| (B) | ||||||||
| Wavelength (nm) | Absorbance and concentration in 80% acetone | Absorbance and concentration in DMSO:80% acetone=1:4 (v/v) mixed solvent | ||||||
| 663.0 | 0.6710±0.0017 | 0.6724±0.0028 | ||||||
| 663.2 | 0.6716±0.0013 | 0.6732±0.0028 | ||||||
| 663.6 | 0.6734±0.0017 | 0.6721±0.0024 | ||||||
| 664.5 | 0.6698±0.0026 | 0.6673±0.0035 | ||||||
| 645.0 | 0.2626±0.0019 | 0.2658±0.0016 | ||||||
| 646.0 | 0.2768±0.0013 | 0.2804±0.0019 | ||||||
| 646.6 | 0.2869±0.0014 | 0.2899±0.0015 | ||||||
| 646.8 | 0.2886±0.0017 | 0.2918±0.0016 | ||||||
| 647.0 | 0.2918±0.0014 | 0.2952±0.0018 | ||||||
| No. | Ca (mg·L-1) | Cb (mg·L-1) | CT (mg·L-1) | Ca/Cb | Ca (mg·L-1) | Cb (mg·L-1) | CT (mg·L-1) | Ca/Cb |
| 1 | 7.82 | 2.87 | 10.69 | 2.72 | 7.82 | 2.94 | 10.76 | 2.66 |
| 2 | 7.85 | 2.87 | 10.73 | 2.73 | 7.86 | 2.94 | 10.81 | 2.67 |
| 3 | 7.72 | 2.80 | 10.53 | 2.75 | 7.68 | 2.89 | 10.57 | 2.66 |
| 4 | 7.42 | 2.20 | 9.61 | 3.38 | 7.42 | 2.26 | 9.68 | 3.28 |
| 5 | 7.42 | 2.78 | 10.20 | 2.67 | 7.43 | 2.84 | 10.27 | 2.62 |
| 6 | 7.52 | 2.52 | 10.04 | 2.98 | 7.49 | 2.59 | 10.08 | 2.90 |
| 7 | - | - | - | - | 7.52 | 2.50 | 10.02 | 3.00 |
Table 4 The calculation formulas (A) and comparison of chlorophyll concentration (B) calculated by different formulas (means±SD)
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | Formulas | References | ||||||
| 1 | Ca (mg·L-1)=12.7A 663-2.69A645; Cb (mg·L-1)=22.9A645-4.68A663 | Arnon, 1949 | ||||||
| 2 | Ca (mg·L-1)=12.72A663-2.59A645; Cb (mg·L-1)=22.88A645-4.67A663 | 陈毓荃, 1986 | ||||||
| 3 | Ca (mg·L-1)=12.63A664.5-2.52A647; Cb (mg·L-1)=20.47A647-4.73A664.5 | Inskeep and Bloom, 1985 | ||||||
| 4 | Ca (mg·L-1)=12.21A663-2.81A646; Cb (mg·L-1)=20.13A646-5.03A663 | Wellburn and Lichtenthaler, 1984 | ||||||
| 5 | Ca (mg·L-1)=12.25A663.2-2.79A646.8; Cb (mg·L-1)=21.50A646.8-5.10A663.2 | Lichtenthaler, 1987 | ||||||
| 6 | Ca (mg·L-1)=12.25A663.6-2.55A646.6; Cb (mg·L-1)=20.31A646.6-4.91A663.6 | Porra et al., 1989 | ||||||
| 7 | Ca (mg·L-1)=12.27A663.6-2.52A646.6; Cb (mg·L-1)=20.10A646.6-4.92A663.6 (correction formula of DMSO:80% acetone=1:4 (v/v) mixed solvent) | - | ||||||
| (B) | ||||||||
| Wavelength (nm) | Absorbance and concentration in 80% acetone | Absorbance and concentration in DMSO:80% acetone=1:4 (v/v) mixed solvent | ||||||
| 663.0 | 0.6710±0.0017 | 0.6724±0.0028 | ||||||
| 663.2 | 0.6716±0.0013 | 0.6732±0.0028 | ||||||
| 663.6 | 0.6734±0.0017 | 0.6721±0.0024 | ||||||
| 664.5 | 0.6698±0.0026 | 0.6673±0.0035 | ||||||
| 645.0 | 0.2626±0.0019 | 0.2658±0.0016 | ||||||
| 646.0 | 0.2768±0.0013 | 0.2804±0.0019 | ||||||
| 646.6 | 0.2869±0.0014 | 0.2899±0.0015 | ||||||
| 646.8 | 0.2886±0.0017 | 0.2918±0.0016 | ||||||
| 647.0 | 0.2918±0.0014 | 0.2952±0.0018 | ||||||
| No. | Ca (mg·L-1) | Cb (mg·L-1) | CT (mg·L-1) | Ca/Cb | Ca (mg·L-1) | Cb (mg·L-1) | CT (mg·L-1) | Ca/Cb |
| 1 | 7.82 | 2.87 | 10.69 | 2.72 | 7.82 | 2.94 | 10.76 | 2.66 |
| 2 | 7.85 | 2.87 | 10.73 | 2.73 | 7.86 | 2.94 | 10.81 | 2.67 |
| 3 | 7.72 | 2.80 | 10.53 | 2.75 | 7.68 | 2.89 | 10.57 | 2.66 |
| 4 | 7.42 | 2.20 | 9.61 | 3.38 | 7.42 | 2.26 | 9.68 | 3.28 |
| 5 | 7.42 | 2.78 | 10.20 | 2.67 | 7.43 | 2.84 | 10.27 | 2.62 |
| 6 | 7.52 | 2.52 | 10.04 | 2.98 | 7.49 | 2.59 | 10.08 | 2.90 |
| 7 | - | - | - | - | 7.52 | 2.50 | 10.02 | 3.00 |
| Methods | Extracted by 80% acetone | Fast two-step extraction | Grinding with 80% acetone | |||||
|---|---|---|---|---|---|---|---|---|
| CT | Ca/Cb | CT | Ca/Cb | CT | Ca/Cb | |||
| Spinach leaf | 8.60±0.22 a | 3.19±0.07 a | 8.50±0.24 a | 3.28±0.10 a | 8.48±0.41 a | 3.12±0.11 a | ||
| Rape leaf | 3.61±0.23 a | 2.74±0.11 a | 3.67±0.21 a | 2.72±0.12 a | 3.55±0.36 a | 2.71±0.14 a | ||
| Poplar leaf | 5.88±0.29 b | 3.57±0.10 a | 7.27±0.19 a | 3.50±0.08 a | 7.24±0.44 a | 3.51±0.15 a | ||
| Chinese pine leaf | 1.19±0.04 b | 3.13±0.06 a | 1.33±0.07 a | 3.17±0.06 a | 1.27±0.11 a | 3.19±0.09 a | ||
Table 5 Comparison of chlorophyll concentration and Ca/Cb extracted and measured by three methods (means±SD)
| Methods | Extracted by 80% acetone | Fast two-step extraction | Grinding with 80% acetone | |||||
|---|---|---|---|---|---|---|---|---|
| CT | Ca/Cb | CT | Ca/Cb | CT | Ca/Cb | |||
| Spinach leaf | 8.60±0.22 a | 3.19±0.07 a | 8.50±0.24 a | 3.28±0.10 a | 8.48±0.41 a | 3.12±0.11 a | ||
| Rape leaf | 3.61±0.23 a | 2.74±0.11 a | 3.67±0.21 a | 2.72±0.12 a | 3.55±0.36 a | 2.71±0.14 a | ||
| Poplar leaf | 5.88±0.29 b | 3.57±0.10 a | 7.27±0.19 a | 3.50±0.08 a | 7.24±0.44 a | 3.51±0.15 a | ||
| Chinese pine leaf | 1.19±0.04 b | 3.13±0.06 a | 1.33±0.07 a | 3.17±0.06 a | 1.27±0.11 a | 3.19±0.09 a | ||
| 1 | 苍晶, 赵会杰 (2013). 植物生理学实验教程. 北京: 高等教育出版社. pp. 57-60. |
| 2 | 陈德海, 徐虹, 连玉武 (2005). 现代植物生物学实验. 北京: 科学出版社. pp. 130-131. |
| 3 | 陈毓荃 (1986). 关于叶绿素的定量测定(分光光度法)中计算系数的修改意见. 西北农业大学学报 14, 84-85. |
| 4 | 段光明 (1992). 叶绿素含量测定中Arnon公式的推导. 植物生理学通讯 28, 221-222. |
| 5 | 高俊凤 (2006). 植物生理学实验指导. 北京: 高等教育出版社. pp. 74-77. |
| 6 | 郝建军, 康宗利, 于洋 (2007). 植物生理学实验技术. 北京: 化学工业出版社. pp. 68-72. |
| 7 | 郝再彬, 苍晶, 徐仲 (2004). 植物生理学实验. 哈尔滨: 哈尔滨工业大学出版社. pp. 46-49. |
| 8 | 侯福林 (2010). 植物生理学实验教程(第2版). 北京: 科学出版社. pp. 61-63. |
| 9 | 胡文玉, 王兴理, 玄英淑 (1984). 二甲基亚砜法测定叶绿素含量. 辽宁农业科学 1, 38-41. |
| 10 | 李合生 (2000). 植物生理生化实验原理与技术. 北京: 高等教育出版社. pp. 134-137. |
| 11 | 舒展, 张晓素, 陈娟, 陈根云, 许大全 (2010). 叶绿素含量测定的简化. 植物生理学通讯 46, 399-402. |
| 12 | 苏正淑, 张宪政 (1989). 几种测定植物叶绿素含量的方法比较. 植物生理学通讯 25(7), 77-78. |
| 13 | 谭桂英, 周百成 (1987). 底栖绿藻叶绿素的二甲基亚砜提取和测定法. 海洋与湖沼 18, 295-300. |
| 14 | 王文杰, 贺海升, 关宇, 李文馨, 张衷华, 祖元刚 (2009). 丙酮和二甲基亚砜法测定植物叶绿素和类胡萝卜素的方法学比较. 植物研究 29, 224-229. |
| 15 | 王英典, 刘宁 (2001). 植物生理学实验指导. 北京: 高等教育出版社. pp. 54-58. |
| 16 | 许大全 (2009). 叶绿素含量的测定及其应用中的几个问题. 植物生理学通讯 45, 896-898. |
| 17 | 张其德 (1985). 测定叶绿素的几种方法. 植物学通报 3(5), 60-64. |
| 18 | 张蜀秋, 李云 (2011). 植物生理学实验技术教程. 北京: 科学出版社. pp. 69-72. |
| 19 | 张志良, 瞿伟菁 (2003). 植物生理学实验指导(第3版). 北京: 高等教育出版社. pp. 67-70. |
| 20 | 邹琦 (2008). 植物生理学实验指导(第2版). 北京: 中国农业出版社. pp. 72-75. |
| 21 | Arnon DI (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24, 1-15. |
| 22 | Hiscox JD, Israesltam GF (1979). A method for extration of chlorophyll from leaf without maceration.Can J Bot 57, 1332-1334. |
| 23 | Inskeep WP, Bloom PR (1985). Extinction coefficients of chlorophyll a and b in N,N-Dimethylformamide and 80% acetone1.Plant Physiol 77, 483-485. |
| 24 | Lichtenthaler HK (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomenbranes. Meth Enzymol 148, 350-382. |
| 25 | Mackinney G (1941). Absorption of light by chlorophyll solution.J Biol Chem 140, 315-322. |
| 26 | Porra RJ (2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b.Photosyn Res 73, 149-156. |
| 27 | Porra RJ, Thompson WA, Kriedemann PE (1989). Deter- mination of accurate extinction coefficients and simultane- ous equations for assaying chlorophylls a and b extra- cted with four different solvents: verification of the con- centration of chlorophyll standards by atomic absorption spectrometry.Biochim Biophys Acta 975, 384-394. |
| 28 | Wellburn AR, Lichtenthaler HK (1984). Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Adv Photosyn Res 2, 9-12. |
| 29 | Wellburn AR (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution.J Plant Physiol 144, 307-313. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||