

Chinese Bulletin of Botany ›› 2015, Vol. 50 ›› Issue (4): 473-481.DOI: 10.11983/CBB14152 cstr: 32102.14.CBB14152
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Huiling Zhao1,2, Limin Lu2, Junxia Su1*, Zhiduan Chen2
Received:2014-08-21
Accepted:2015-01-27
Online:2015-07-01
Published:2015-05-07
Contact:
Su Junxia
About author:? These authors contributed equally to this paper
Huiling Zhao, Limin Lu, Junxia Su, Zhiduan Chen. Phylogeny of Stixeae and Borthwickiaceae Based on Morphological and Molecular Data[J]. Chinese Bulletin of Botany, 2015, 50(4): 473-481.
| Taxon | Locality | Voucher |
|---|---|---|
| Borthwickia trifoliata W.W. Smith | Mengla, Yunnan | RS002 |
| Stixis suaveolens (Roxb.) Pierre | Mengla, Yunnan | RS012 |
| Stixis ovata subsp. fasciculata (King) Jacobs | Hekou, Yunnan | RS019 |
| Stixis scandens Lour. | Mengla, Yunnan | RS013 |
Table 1 Origin of materials
| Taxon | Locality | Voucher |
|---|---|---|
| Borthwickia trifoliata W.W. Smith | Mengla, Yunnan | RS002 |
| Stixis suaveolens (Roxb.) Pierre | Mengla, Yunnan | RS012 |
| Stixis ovata subsp. fasciculata (King) Jacobs | Hekou, Yunnan | RS019 |
| Stixis scandens Lour. | Mengla, Yunnan | RS013 |

Figure 1 Characters of the leaf epidermis under LM and SEM in Borthwickia and Stixis (A)-(D) Adaxial epidermis and anticlinal wall; (E)-(H) Characters of the stomata; (I), (J) Cuticular membrane; (K), (L) Trichome. (A), (E), (I), (L) Borthwickia trifoliata; (B), (F) Stixis suaveolens; (D), (H) Stixis ovata subsp. fasciculata; (C), (G), (J), (K) Stixis scandens. Bar=100 µm
| matK | ndhF | rbcL | trnL-F | ITS | Five loci data | Morphological data | Total evidence | |
|---|---|---|---|---|---|---|---|---|
| Total length | 1 320 | 923 | 1 270 | 1 122 | 689 | 5 012 | 20 | 5 032 |
| Variable characters | 306 | 180 | 126 | 159 | 381 | 1 032 | 20 | 1 266 |
| Informative characters | 212 | 145 | 90 | 89 | 326 | 760 | 20 | 780 |
| Number of trees | 11 415 | 12 780 | 10 | 12 045 | 288 | 32 | 210 | 20 |
| Length of trees | 398 | 235 | 163 | 192 | 949 | 1 410 | 56 | 1 756 |
| Consistency index | 0.884 | 0.872 | 0.853 | 0.911 | 0.624 | 0.859 | 0.875 | 0.876 |
| Retention index | 0.946 | 0.942 | 0.938 | 0.953 | 0.887 | 0.938 | 0.963 | 0.936 |
| Rescaled consistency index | 0.837 | 0.822 | 0.799 | 0.869 | 0.553 | 0.806 | 0.842 | 0.820 |
Table 2 Statistics from phylogenetic analyses of the various datasets
| matK | ndhF | rbcL | trnL-F | ITS | Five loci data | Morphological data | Total evidence | |
|---|---|---|---|---|---|---|---|---|
| Total length | 1 320 | 923 | 1 270 | 1 122 | 689 | 5 012 | 20 | 5 032 |
| Variable characters | 306 | 180 | 126 | 159 | 381 | 1 032 | 20 | 1 266 |
| Informative characters | 212 | 145 | 90 | 89 | 326 | 760 | 20 | 780 |
| Number of trees | 11 415 | 12 780 | 10 | 12 045 | 288 | 32 | 210 | 20 |
| Length of trees | 398 | 235 | 163 | 192 | 949 | 1 410 | 56 | 1 756 |
| Consistency index | 0.884 | 0.872 | 0.853 | 0.911 | 0.624 | 0.859 | 0.875 | 0.876 |
| Retention index | 0.946 | 0.942 | 0.938 | 0.953 | 0.887 | 0.938 | 0.963 | 0.936 |
| Rescaled consistency index | 0.837 | 0.822 | 0.799 | 0.869 | 0.553 | 0.806 | 0.842 | 0.820 |
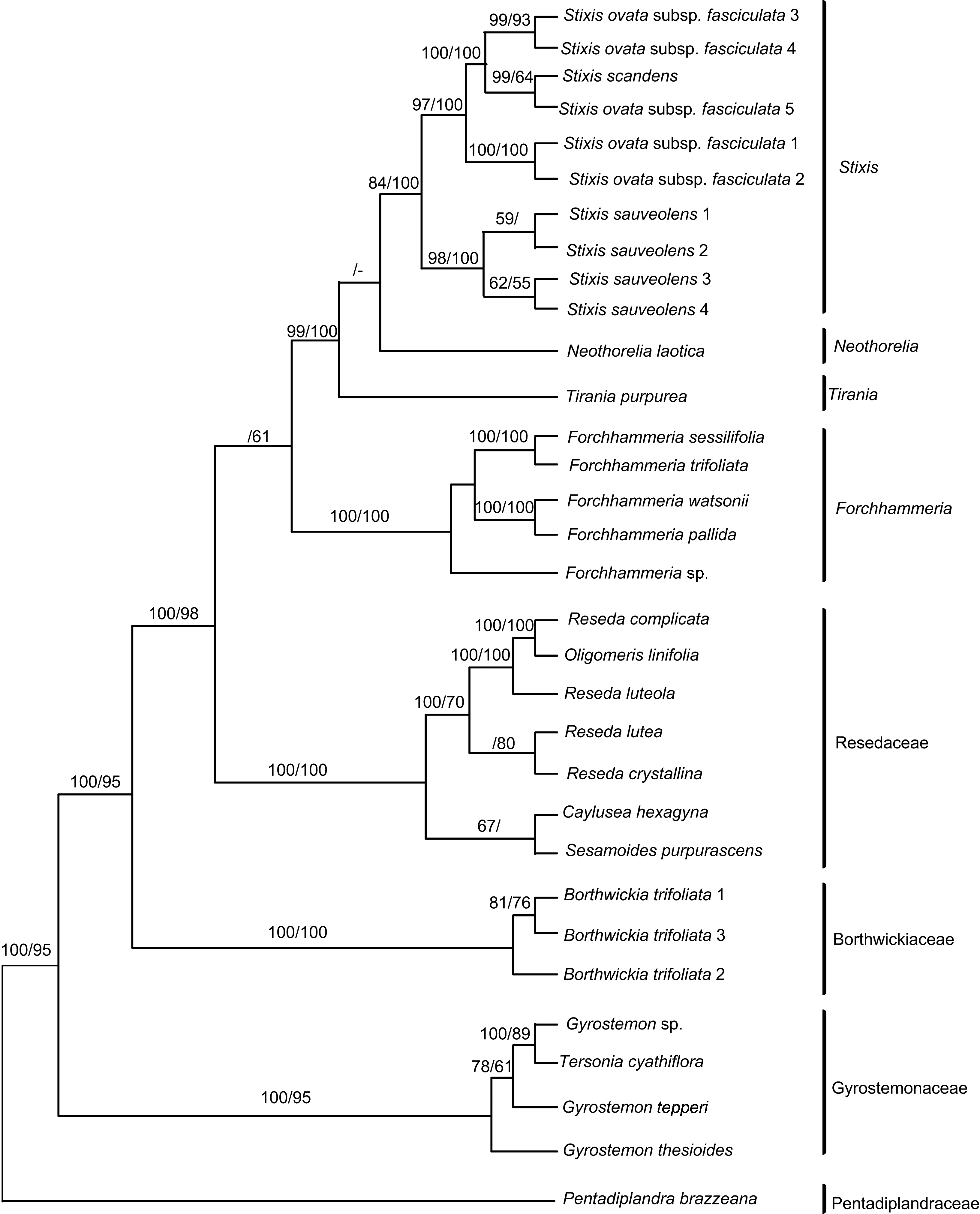
Figure 2 Phylogram of Bayesian inference (BI) tree based on analysis of 32-taxon, 5-locus data and morphological data set Numbers on branches indicate the BI pp and MP BS; dash indicates different topological structure on the node.
| Taxon | Voucher | ITS | matK | ndhF | rbcL | trnL-F |
|---|---|---|---|---|---|---|
| Borthwickia trifoliata 1 W.W. Smith | SJX036 | KR936124* | JQ733089 | JQ733101 | JQ733114 | JQ733127 |
| Borthwickia trifoliata 2 W.W. Smith | Jin & al. YNET 381 | KR936122* | JQ733091 | JQ733103 | JQ733116 | JQ733129 |
| Borthwickia trifoliata 3 W.W. Smith | RS002 | KR936123* | JQ733090 | JQ733102 | JQ733115 | JQ733128 |
| Caylusea hexagyna (Forssk.) M.L. Green | DQ987228 | FJ212207 | - | FJ212220 | DQ987069 | |
| Forchhammeria trifoliata Radlk. | - | AY483245 | AY483259 | AY483277 | - | |
| Forchhammeria sessilifolia Standl. | - | AY483243, | AY483257 | AY483275 | - | |
| Forchhammeria sp. | - | AY483244 | AY483258 | AY483276 | - | |
| Forchhammeria watsonii Rose | - | AY483246 | AY483260 | AY483278 | - | |
| Forchhammeria pallida Liebm. | - | - | AY122381 | AY483274 | AY122437 | |
| Gyrostemon sp. | - | AY483236 | AY483252 | L22439 | - | |
| Gyrostemon tepperi (F. Muell. ex H. Walter) A.S. George | - | AY483237 | AY483253 | L22440 | - | |
| Gyrostemon thesioides (Hook. f.) A.S. George | DQ987075 | FJ212199 | - | FJ212210 | DQ986975 | |
| Oligomeris linifolia (Vahl) J.F. Macbr. | DQ987162 | AY483240 | AY483255 | AY483272 | FJ212256 | |
| Pentadiplandra brazzeana Baill. | - | AY483239 | AY483254 | U38533 | AY122463 | |
| Reseda complicata Bory | DQ987171 | FJ212205 | - | FJ212218 | DQ987046 | |
| Reseda crystallina Webb & Berthel | DQ987089 | FJ212200 | - | FJ212212 | FJ212283 | |
| Reseda lutea L. | M. Merello, J. Stone, M. Eristavi & M. Khutsishvili 2211 | KR936125* | AY483241 | AY122406 | AY483273 | AY122464 |
| Reseda luteola L. | DQ987171 | FJ212206 | - | FJ212219 | DQ987050 | |
| Sesamoides purpurascens (L.) G. López. | DQ987216 | FJ212208 | - | FJ212221 | DQ987064 | |
| Stixis sauveolens 1 (Roxb.) Pierre | BW04 | KR936112* | JQ733092 | JQ733104 | JQ733117 | JQ733130 |
| Stixis sauveolens 2 (Roxb.) Pierre | RS012 | KR936113* | JQ733094 | JQ733106 | JQ733119 | JQ733131 |
| Stixis sauveolens 3 (Roxb.) Pierre | RS015 | KR936114* | JQ733096 | JQ733108 | JQ733121 | JQ733132 |
| Stixis sauveolens 4 (Roxb.) Pierre | Chen Z.D. HN171 | KR936115* | JQ733100 | JQ733113 | JQ733126 | JQ733133 |
| Stixis ovata subsp. fasciculata 1 (King) Jacobs | RS001 | KR936117* | JQ733093 | JQ733105 | JQ733118 | JQ733134 |
| Stixis ovata subsp. fasciculata 2 (King) Jacobs | RS013 | KR936116* | JQ733095 | JQ733107 | JQ733120 | JQ733135 |
| Stixis ovata subsp. fasciculata 3 (King) Jacobs | RS017 | KR936120* | JQ733097 | JQ733109 | JQ733122 | JQ733136 |
| Stixis ovata subsp. fasciculata 4 (King) Jacobs | RS019 | KR936121* | JQ733098 | JQ733110 | JQ733123 | JQ733137 |
| Stixis ovata subsp. fasciculata 5 (King) Jacobs | Yang Z.G. 008 | KR936119* | - | JQ733112 | JQ733125 | JQ733139 |
| Stixis scandens Lour. | Shui et al.12443 | KR936118* | JQ733099 | JQ733111 | JQ733124 | JQ733138 |
| Tersonia cyathiflora (Fenzl) A.S. George ex J.W. | - | AY483238 | AY122404 | L22441 | AY122462 | |
| Tirania purpurea Pierre | - | - | AY483261 | AY483279 | - |
Appendix 1 Taxa, voucher information and GenBank accession numbers for all samples included in this study
| Taxon | Voucher | ITS | matK | ndhF | rbcL | trnL-F |
|---|---|---|---|---|---|---|
| Borthwickia trifoliata 1 W.W. Smith | SJX036 | KR936124* | JQ733089 | JQ733101 | JQ733114 | JQ733127 |
| Borthwickia trifoliata 2 W.W. Smith | Jin & al. YNET 381 | KR936122* | JQ733091 | JQ733103 | JQ733116 | JQ733129 |
| Borthwickia trifoliata 3 W.W. Smith | RS002 | KR936123* | JQ733090 | JQ733102 | JQ733115 | JQ733128 |
| Caylusea hexagyna (Forssk.) M.L. Green | DQ987228 | FJ212207 | - | FJ212220 | DQ987069 | |
| Forchhammeria trifoliata Radlk. | - | AY483245 | AY483259 | AY483277 | - | |
| Forchhammeria sessilifolia Standl. | - | AY483243, | AY483257 | AY483275 | - | |
| Forchhammeria sp. | - | AY483244 | AY483258 | AY483276 | - | |
| Forchhammeria watsonii Rose | - | AY483246 | AY483260 | AY483278 | - | |
| Forchhammeria pallida Liebm. | - | - | AY122381 | AY483274 | AY122437 | |
| Gyrostemon sp. | - | AY483236 | AY483252 | L22439 | - | |
| Gyrostemon tepperi (F. Muell. ex H. Walter) A.S. George | - | AY483237 | AY483253 | L22440 | - | |
| Gyrostemon thesioides (Hook. f.) A.S. George | DQ987075 | FJ212199 | - | FJ212210 | DQ986975 | |
| Oligomeris linifolia (Vahl) J.F. Macbr. | DQ987162 | AY483240 | AY483255 | AY483272 | FJ212256 | |
| Pentadiplandra brazzeana Baill. | - | AY483239 | AY483254 | U38533 | AY122463 | |
| Reseda complicata Bory | DQ987171 | FJ212205 | - | FJ212218 | DQ987046 | |
| Reseda crystallina Webb & Berthel | DQ987089 | FJ212200 | - | FJ212212 | FJ212283 | |
| Reseda lutea L. | M. Merello, J. Stone, M. Eristavi & M. Khutsishvili 2211 | KR936125* | AY483241 | AY122406 | AY483273 | AY122464 |
| Reseda luteola L. | DQ987171 | FJ212206 | - | FJ212219 | DQ987050 | |
| Sesamoides purpurascens (L.) G. López. | DQ987216 | FJ212208 | - | FJ212221 | DQ987064 | |
| Stixis sauveolens 1 (Roxb.) Pierre | BW04 | KR936112* | JQ733092 | JQ733104 | JQ733117 | JQ733130 |
| Stixis sauveolens 2 (Roxb.) Pierre | RS012 | KR936113* | JQ733094 | JQ733106 | JQ733119 | JQ733131 |
| Stixis sauveolens 3 (Roxb.) Pierre | RS015 | KR936114* | JQ733096 | JQ733108 | JQ733121 | JQ733132 |
| Stixis sauveolens 4 (Roxb.) Pierre | Chen Z.D. HN171 | KR936115* | JQ733100 | JQ733113 | JQ733126 | JQ733133 |
| Stixis ovata subsp. fasciculata 1 (King) Jacobs | RS001 | KR936117* | JQ733093 | JQ733105 | JQ733118 | JQ733134 |
| Stixis ovata subsp. fasciculata 2 (King) Jacobs | RS013 | KR936116* | JQ733095 | JQ733107 | JQ733120 | JQ733135 |
| Stixis ovata subsp. fasciculata 3 (King) Jacobs | RS017 | KR936120* | JQ733097 | JQ733109 | JQ733122 | JQ733136 |
| Stixis ovata subsp. fasciculata 4 (King) Jacobs | RS019 | KR936121* | JQ733098 | JQ733110 | JQ733123 | JQ733137 |
| Stixis ovata subsp. fasciculata 5 (King) Jacobs | Yang Z.G. 008 | KR936119* | - | JQ733112 | JQ733125 | JQ733139 |
| Stixis scandens Lour. | Shui et al.12443 | KR936118* | JQ733099 | JQ733111 | JQ733124 | JQ733138 |
| Tersonia cyathiflora (Fenzl) A.S. George ex J.W. | - | AY483238 | AY122404 | L22441 | AY122462 | |
| Tirania purpurea Pierre | - | - | AY483261 | AY483279 | - |
| No. | Morphological characters |
|---|---|
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 | Phyllotaxy: Alternate (0); Opposite (1) Habit: Shrub (0); Tree (1); Woody vine (2); Annual herb (3); Perennial herb (4) Division of leaves: Unifoliate (0); Palmate (1); 3 leaflets (2) Stipule: Absent (0); Present (1) Leaf epidermal cells: Polygonal (0); Irregular (1) Axillary inflorescence: Present (0); Absent (1) Breeding system: Monoecious (0); Dioecious (1) Inflorescence: Raceme (0); Panicle (1) Calyx: Persistent (0); Caducous (1) Style: Persistent (0); Caducous (1) Petal: 6 (0); 0 (1); 5–8 (2); 4–9 (3); 6–8 (4) Sepal: 6 (0); 4–9 (1); 5–8 (2); 2–5 (3); 6–8 (4) Stigma: 3 (0); ≥2 (1); 1 (2) Carpel: 3 (0); 2 (1); 4–6 (2); 0 (3) Locule: 3 (0); 2 (1); 4–8 (3); 1 (4) Ovary: Spherical (0); Pyriform (1); Linear (2); Quadrilateral (3); Turbinate (4) Placentation: Axile (0); Parietal (1) Pollen exine ornamentation: Reticulate (0); Cave-shaped (1); Rugulate (2); Spinulate (3) Pollen grain shape: Spherical (0); Subprolate (1); Prolate (2) Fruit shape: Subsphaeroidal (0); Spherical (1); Elliptoid (2); Linear (3); Capsule (4); Turbinate (5) |
Appendix 2 Morphological characters scored for phylogenetic analysis
| No. | Morphological characters |
|---|---|
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 | Phyllotaxy: Alternate (0); Opposite (1) Habit: Shrub (0); Tree (1); Woody vine (2); Annual herb (3); Perennial herb (4) Division of leaves: Unifoliate (0); Palmate (1); 3 leaflets (2) Stipule: Absent (0); Present (1) Leaf epidermal cells: Polygonal (0); Irregular (1) Axillary inflorescence: Present (0); Absent (1) Breeding system: Monoecious (0); Dioecious (1) Inflorescence: Raceme (0); Panicle (1) Calyx: Persistent (0); Caducous (1) Style: Persistent (0); Caducous (1) Petal: 6 (0); 0 (1); 5–8 (2); 4–9 (3); 6–8 (4) Sepal: 6 (0); 4–9 (1); 5–8 (2); 2–5 (3); 6–8 (4) Stigma: 3 (0); ≥2 (1); 1 (2) Carpel: 3 (0); 2 (1); 4–6 (2); 0 (3) Locule: 3 (0); 2 (1); 4–8 (3); 1 (4) Ovary: Spherical (0); Pyriform (1); Linear (2); Quadrilateral (3); Turbinate (4) Placentation: Axile (0); Parietal (1) Pollen exine ornamentation: Reticulate (0); Cave-shaped (1); Rugulate (2); Spinulate (3) Pollen grain shape: Spherical (0); Subprolate (1); Prolate (2) Fruit shape: Subsphaeroidal (0); Spherical (1); Elliptoid (2); Linear (3); Capsule (4); Turbinate (5) |
| Taxon | The number of morphological characters | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Neothorelia laotica Gagnep. | 0 | 0 | 2 | 0 | ? | ? | ? | 1 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | ? |
| Tirania purpurea Pierre | 0 | 0 | 0 | 1 | ? | 0 | 0 | 0 | ? | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | ? | 0 | 1 |
| Stixis sauveolens 1 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis sauveolens 2 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis sauveolens 3 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis sauveolens 4 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 1 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 2 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 3 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 4 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 5 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis scandens Lour | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Borthwickia trifoliata 1 W.W. Smith | 1 | 0/1 | 1 | 0 | 1 | 1 | 0 | ? | 1 | ? | 2 | 2 | 2 | 2 | 3 | 2 | 0 | 1 | 1 | 3 |
| Borthwickia trifoliata 2 W.W. Smith | 1 | 0/1 | 1 | 0 | 1 | 1 | 0 | ? | 1 | ? | 2 | 2 | 2 | 2 | 3 | 2 | 0 | 1 | 1 | 3 |
| Borthwickia trifoliata 3 W.W. Smith | 1 | 0/1 | 1 | 0 | 1 | 1 | 0 | ? | 1 | ? | 2 | 2 | 2 | 2 | 3 | 2 | 0 | 1 | 1 | 3 |
| Reseda complicata Bory | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Reseda luteola L. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Reseda crystallina Webb & Berthel. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Caylusea hexagyna (Forssk.) M.L. Green | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 3 | 4 | 3 | 1 | 2 | 2 | 4 |
| Sesamoides purpurascens (L.) G. López. | 1 | 4 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | ? | 4 | 3 | 1 | ? | 2 | 4 |
| Oligomeris linifolia (Vahl) J.F. Macbr. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 2 | 3 | 1 | 2 | 4 | 3 | 1 | 0 | 2 | 4 |
| Reseda lutea L. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Gyrostemon sp. | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| Gyrostemon tepperi (F. Muell. ex H. Walter) A.S. George | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| Gyrostemon thesioides (Hook. f.) A.S. George | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| Forchhammeria trifoliata Radlk. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria sessilifolia Standl. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria sp. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria watsonii Rose | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria pallida Liebm. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pentadiplandra brazzeana Baill. | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | ? | 0 | 4 | 5 | 1 | ? | ? | ? | ? | 0 | 2 | 1 |
| Tersonia cyathiflora (Fenzl) A.S. George ex J.W. | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
Appendix 2 Morphological data matrix
| Taxon | The number of morphological characters | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| Neothorelia laotica Gagnep. | 0 | 0 | 2 | 0 | ? | ? | ? | 1 | ? | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | ? |
| Tirania purpurea Pierre | 0 | 0 | 0 | 1 | ? | 0 | 0 | 0 | ? | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | ? | 0 | 1 |
| Stixis sauveolens 1 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis sauveolens 2 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis sauveolens 3 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis sauveolens 4 (Roxb.) Pierre | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 1 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 2 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 3 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 4 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis ovata subsp. fasciculata 5 (King) Jacobs | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Stixis scandens Lour | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Borthwickia trifoliata 1 W.W. Smith | 1 | 0/1 | 1 | 0 | 1 | 1 | 0 | ? | 1 | ? | 2 | 2 | 2 | 2 | 3 | 2 | 0 | 1 | 1 | 3 |
| Borthwickia trifoliata 2 W.W. Smith | 1 | 0/1 | 1 | 0 | 1 | 1 | 0 | ? | 1 | ? | 2 | 2 | 2 | 2 | 3 | 2 | 0 | 1 | 1 | 3 |
| Borthwickia trifoliata 3 W.W. Smith | 1 | 0/1 | 1 | 0 | 1 | 1 | 0 | ? | 1 | ? | 2 | 2 | 2 | 2 | 3 | 2 | 0 | 1 | 1 | 3 |
| Reseda complicata Bory | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Reseda luteola L. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Reseda crystallina Webb & Berthel. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Caylusea hexagyna (Forssk.) M.L. Green | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 3 | 4 | 3 | 1 | 2 | 2 | 4 |
| Sesamoides purpurascens (L.) G. López. | 1 | 4 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | ? | 4 | 3 | 1 | ? | 2 | 4 |
| Oligomeris linifolia (Vahl) J.F. Macbr. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 2 | 3 | 1 | 2 | 4 | 3 | 1 | 0 | 2 | 4 |
| Reseda lutea L. | 1 | 3 | 0 | 1 | ? | 1 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 0 | 4 | 3 | 1 | 2 | 2 | 4 |
| Gyrostemon sp. | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| Gyrostemon tepperi (F. Muell. ex H. Walter) A.S. George | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| Gyrostemon thesioides (Hook. f.) A.S. George | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| Forchhammeria trifoliata Radlk. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria sessilifolia Standl. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria sp. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria watsonii Rose | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Forchhammeria pallida Liebm. | 0 | 1 | 0 | 0 | ? | ? | 1 | 0 | 0 | ? | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Pentadiplandra brazzeana Baill. | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | ? | 0 | 4 | 5 | 1 | ? | ? | ? | ? | 0 | 2 | 1 |
| Tersonia cyathiflora (Fenzl) A.S. George ex J.W. | 0 | 0 | 0 | 1 | ? | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 1 | 0 | 0 | 3 | 2 | 6 |
| 1 | 洪亚平, 潘开玉, 陈之端, 路安民 (2001). 防己科植物的叶表皮特征及其系统学意义. 植物学报 43, 615-623. |
| 2 | 桑涛, 徐炳声 (1996). 分支系统学当前的理论和方法概述及华东地区山胡椒属十二个种的分支系统学研究. 植物分类学报 34, 12-28. |
| 3 | 苏俊霞 (2012). 蔷薇分支的分子系统学研究. 博士学位论文. 北京: 中国科学院植物研究所. |
| 4 | 孙必兴 (1999). 山柑科. 见: 吴征镒编. 中国植物志(第32卷). 北京: 科学出版社. pp. 484-540. |
| 5 | 孙稚颖, 张宪春 (2009). 中国瓦韦属药用植物的叶表皮观察. 植物学报 44, 331-337. |
| 6 | 吴征镒, 路安民, 汤彦承, 陈之端, 李德铢 (2004). 中国被子植物科属综论. 北京: 科学出版社. |
| 7 | APG III (Angiosperm Phylogeny Group III). (2009). An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III.Bot J Linn Soc 161, 105-121. |
| 8 | Carlquist S, Hansen BF, Iltis HH, Olson ME, Geiger DL (2014). Forchhammeria and Stixis (Brassicales): stem and wood anatomical diversity, ecological and phylogenetic significance.Aliso 31, 59-75. |
| 9 | Dogan Y, Mert H, Akcan K (2008). Anatomical studies of Reseda lutea (Resedaceae).Phytologia Balcan 14, 91-95. |
| 10 | Dowled A, Reveal JL (2008). New suprageneric names for vascular plants.Phytologia 90, 416-417. |
| 11 | Erdtman G (1952). Pollen Morphology and Plant Taxonomy. Angiosperms. Stockholm: Almquist and Wiksell. |
| 12 | Hansen BF (1977). A Monograph of Forchhammeria (Capparidaceae). Master Thesis Dissertation, University of Wisconsin, Madison, Wisconsin, USA. |
| 13 | Hall JC, Iltis HH, Sytsma KJ (2004). Molecular phylogenetics of core Brassicales, placement of orphan genera Emblingia, Forchhammeria, Tirania, and character evolution.Syst Bot 29, 654-669. |
| 14 | Hall JC, Sytsma KJ, Iltis HH (2002). Phylogeny of Capparaceae and Brassicaceae based on chloroplast sequence data.Am J Bot 89, 1826-1842. |
| 15 | Hillis DM, Bull JJ (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis.Syst Biol 42, 182-192. |
| 16 | Hutchinson J (1967). The genera of flowering plants (Angiospermae), Vol. 2. Dicotyledons. Oxford: Clarendon Press. |
| 17 | Jacobs M (1968). Borthwickia (Capparaceae) from Yunnan and in fruit.Blumea 16, 360. |
| 18 | Kers LE (2003). Capparaceae. In: Kubitzki K, Bayer C, eds. The Families and Genera of Vascular Plants, Vol. 5. Flowering Plants: Dicotyledons; Malvales, Capparales, and non-betalain Caryophyllales. Berlin: Springer. pp. 36-56. |
| 19 | Kubitzki K (2003). Resedaceae. In: Kubitzki K, Bayer C, eds. The Families and Genera of Vascular Plants, Vol. 5. Flowering Plants: Dicotyledons; Malvales, Capparales, and non-betalain Caryophyllales. Berlin: Springer. pp. 334-338. |
| 20 | Mabberley DJ (2008). Mabberley’s plant-book, third edn. Cambridge: Cambridge University Press. |
| 21 | Martín-Bravo S, Meimberg H, Luceño M, Märkl W, Valcárcel V, Bräuchler C, Vargas P, Heubl G (2007). Molecular systematics and biogeography of Resedaceae based on ITS and trnL-F sequences.Mol Phylogenet Evol 44, 1105-1120. |
| 22 | Mitra K (1975). Contribution to the pollen morphology of the family Capparaceae.Bull Bot Surv India 17, 7-31. |
| 23 | Morton CM, Karol KG, Chase MW (1997). Taxonomic affinities of Physena (Physenaceae) and Asteropeia (Theaceae).Bot Rev 63, 231-239. |
| 24 | Pax F, Hoffmann K (1936). Capparidaceae. In: Engler A, Prantl K, eds. Die natürlichen Pflanzenfamilien, Vol. 17b. Leipzig: Engelmann. pp. 146-233. |
| 25 | Ronquist F, Huelsenbeck JP (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models.Bioinformatics 19, 1572-1574. |
| 26 | Ronse De Craene LP, Haston E (2006). The systematic relationships of glucosinolate-producing plants and related families: a cladistic investigation based on morphological and molecular characters.Bot J Linn Soc 151, 453-494. |
| 27 | Silvio F (2010). Micromorphological observations on leaf and pollen of Capparis L. sect. Capparis (Capparaceae).Plant Biosyst 138, 125-134. |
| 28 | Smith WW (1911). Borthwickia, a new genera of Cappari- daceae.Trans Proc Bot Soc Edinburgh 24, 175-176. |
| 29 | Stanford AM, Harden M, Parks CR (2000). Phylogeny and biogeography of Juglans (Juglanaceae) based on matK and ITS sequence data. Am J Bot 87, 872-882. |
| 30 | Swofford DL (2003). PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sunderland, Massachusetts: Sinauer. |
| 31 | Su JX, Wang W, Zhang LB, Chen ZD (2012). Phylogenetic placement of two enigmatic genera, Borthwickia and Stixis, based on molecular and pollen data, and the description of a new family of Brassicales, Borthwicki- aceae.Taxon 61, 601-611. |
| 32 | Takhtajan A (1997). Diversity and Classification of Flowering Plants. New York: Columbia University Press. |
| 33 | White TJ, Bruns T, Lee S, Taylor J (1990). Amplifications and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, eds. PCR Protocols: A Guide to Methods and Applications. San Diego, California: Academic Press. pp. 315-322. |
| 34 | Zhang ML, Tucker GC (2008). Capparaceae. In: Wu ZY, Raven PH, Hong DY, eds. Flora of China, Vol. 7. Beijing: Science Press; St. Louis: Missouri Botanical Garden. pp. 433-450. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||