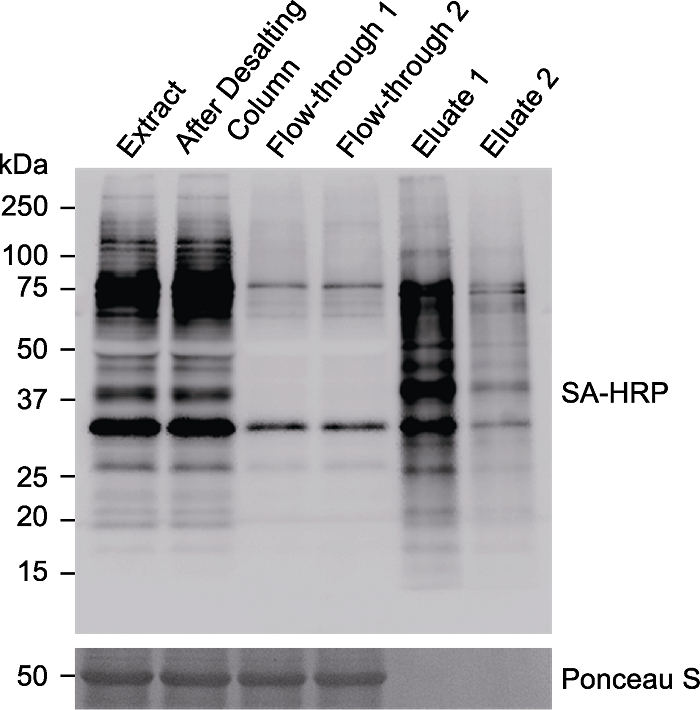
图5. 免疫印迹检测被磁珠富集的生物素化蛋白
对表达TurboID-EYFP-ATG8a的拟南芥植株进行生物素处理(50 µmol∙L-1, 3小时), 提取蛋白后使用脱盐柱进行蛋白脱盐, 将脱盐后的样品等体积分成2份, 分别与20 μL Streptavidin Magnetic Beads过夜孵育, 最后分别用20 μL或200 μL 4× SDS Sample Buffer进行洗脱。Extract: 离心后的蛋白上清; After Desalting Column: 脱盐后的蛋白样品; Flow-through 1/2: 与链霉亲和素磁珠过夜孵育后的上清; Eluate 1: 使用20 μL buffer洗脱的蛋白样品; Eluate 2: 使用200 μL buffer洗脱的蛋白样品; SA-HRP: 辣根过氧化物酶标记链霉亲和素
Figure 5. Immunoblotting analyses of the biotinylated proteins enriched by Streptavidin Magnetic Beads
Arabidopsis seedlings overexpressing TurboID-EYFP-ATG8a were treated with 50 µmol∙L-1 biotin for 3 hours. The protein was extracted and desalted through Desalting Column. The desalted sample was split into two parts and incubated with 20 μL Streptavidin Magnetic Beads overnight, followed by protein elution with 20 μL or 200 μL 4× SDS Sample Buffer, respectively. Extract: Protein supernatant after centrifugation; After Desalting Column: Protein sample after desalting; Flow-through 1 or 2: Supernatant after overnight incubation with streptavidin (SA) beads; Eluate 1: Protein sample eluated with 20 μL buffer; Eluate 2: Protein sample eluated with 200 μL buffer; SA-HRP: Streptavidin-HRP