

Quantitative Analysis of Plasma Membrane Order in Live Plant Cells
Received date: 2024-03-11
Accepted date: 2024-05-27
Online published: 2024-05-30
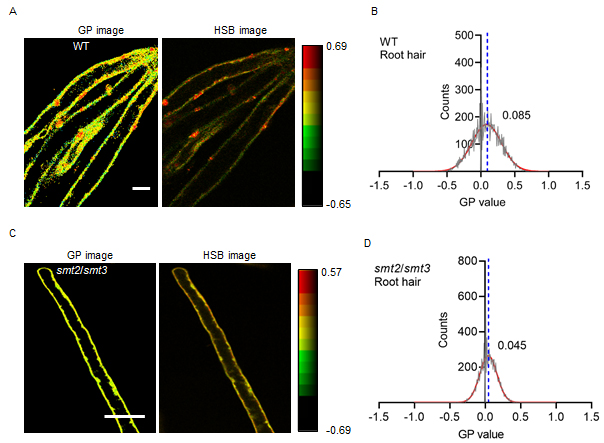
Membrane microdomains, which are highly dynamic structures rich in sterols and sphingolipids on the plasma membrane, play crucial roles in various biological processes such as signal transduction, vesicle transport, endocytosis, and exocytosis. Consequently, the investigation of membrane microdomain dynamics is an important area of research in plant cell biology. Fluorescence probes combined with fluorescence microscopy are widely used to monitor the status of living plant cells. The PA probe (push-pull pyrene) is a novel, highly efficient and stable fluorescence probe based on pyrene: however, its application in imaging studies of living plant cells is limited. In this study, we used PA probes and laser scanning confocal microscopy, combined with image processing and the polar normalized value mapping method, to quantitatively analyze the order of the plasma membrane in Arabidopsis root cells. The results revealed that the emission spectrum of the liquid-ordered phase in the plasma membrane of Arabidopsis root cells labeled with the PA probe ranged from 500-550 nm, whereas the emission spectrum of the liquid-disordered phase ranged from 580-700 nm. Treatment of wild-type plants with the sterol extraction agent MβCD resulted in a decrease in plasma membrane order. In the smt2/smt3 double mutant lacking the key methyltransferase in sterol synthesis, the plasma membrane order was consistent with that of the wild-type plants after treatment with MβCD. In the smt2/smt3 mutant, the plasma membrane order of the root hair cells was lower than the plasma membrane order of the wild-type root hair cells, indicating that sterols, as key components of membrane microdomains, play an important role in regulating the order of the plasma membrane. This study provides a straightforward and rapid detection method for monitoring the dynamic characteristics of living plant cell membranes and changes in membrane microdomains.
Xiuxiu Chen , Ling Tang , Wenjia Hu , Zhaolin Yang , Xin Deng , Xiaohua Wang . Quantitative Analysis of Plasma Membrane Order in Live Plant Cells[J]. Chinese Bulletin of Botany, 2025 , 60(1) : 90 -100 . DOI: 10.11983/CBB24040
| [1] | Brown DA (1992). Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol 2, 338-343. |
| [2] | Brown DA, London E (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275, 17221-17224. |
| [3] | Carland F, Fujioka S, Nelson T (2010). The sterol methyltransferases SMT1, SMT2, and SMT3 influence Arabidopsis development through nonbrassinosteroid products. Plant Physiol 153, 741-756. |
| [4] | Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T (2002). The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 14, 2045-2058. |
| [5] | Chiantia S, Ries J, Kahya N, Schwille P (2006). Combined AFM and two-focus SFCS study of raft-exhibiting model membranes. Chem Phys Chem 7, 2409-2418. |
| [6] | Dong ZY, Song CW, Cui YN, Yu M, Li RL, Lin JX (2019). Structural models of membrane microdomains and sterol imaging technology. J Chin Electron Microsc Soc 38, 542-549. (in Chinese) |
| 董紫怡, 宋程威, 崔亚宁, 玉猛, 李瑞丽, 林金星 (2019). 膜微区相关结构模型及甾醇成像技术的研究进展. 电子显微学报 38, 542-549. | |
| [7] | Filippov A, Or?dd G, Lindblom G (2003). The effect of cholesterol on the lateral diffusion of phospholipids in oriented bilayers. Biophys J 84, 3079-3086. |
| [8] | Gerke V, Gavins FNE, Geisow M, Grewal T, Jaiswal JK, Nylandsted J, Rescher U (2024). Annexins—a family of proteins with distinctive tastes for cell signaling and mem-brane dynamics. Nat Commun 15, 1574. |
| [9] | Goksu EI, Vanegas JM, Blanchette CD, Lin WC, Longo ML (2009). AFM for structure and dynamics of biomembranes. Biochim Biophys Acta (BBA) Biomembr 1788,254-266. |
| [10] | Hoppe T, Rape M, Jentsch S (2001). Membrane-bound transcription factors: regulated release by RIP or RUP. Curr Opin Cell Biol 13, 344-348. |
| [11] | Huang XH, Liu W, Tian SP, Chen T (2023). Advances in the regulation of protein liquid-liquid phase separation on development and stress responses in plants. Chin Bull Bot 58, 946-955. (in Chinese) |
| 黄鑫华, 刘伟, 田世平, 陈彤 (2023). 蛋白液-液相分离调控植物发育及胁迫应答研究进展. 植物学报 58, 946-955. | |
| [12] | Ivanov S, Harrison MJ (2024). Receptor-associated kinases control the lipid provisioning program in plant-fungal symbiosis. Science 383, 443-448. |
| [13] | Jin L, Millard AC, Wuskell JP, Clark HA, Loew LM (2005). Cholesterol-enriched lipid domains can be visualized by di-4-ANEPPDHQ with linear and nonlinear optics. Biophys J 89, L04-L06. |
| [14] | Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, Kasai RS, Suzuki KGN (2012). Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of singer and nicolson's fluid-mosaic model. Annu Rev Cell Dev Biol 28, 215-250. |
| [15] | Lingwood D, Simons K (2010). Lipid rafts as a membrane-organizing principle. Science 327, 46-50. |
| [16] | Luo PY, Qian HP, Liu Y, Xu CW, Cui YN (2023). Regulation of plasma membrane protein dynamics and its research methods. Chin Bull Bot 58, 590-601. (in Chinese) |
| 罗鹏云, 钱虹萍, 刘艳, 徐昌文, 崔亚宁 (2023). 质膜蛋白动力学的调控及其研究方法. 植物学报 58, 590-601. | |
| [17] | Lv XQ, Jin K, Liu JH, Cui SX, Li JH, Du GC, Liu L (2021). Quantitative analysis of membrane ordering of living industrial model microorganisms. China Biotechnol 41, 20-29. (in Chinese) |
| 吕雪芹, 金柯, 刘家恒, 崔世修, 李江华, 堵国成, 刘龙 (2021). 工业模式微生物膜有序性的活细胞定量分析. 中国生物工程杂志 41, 20-29. | |
| [18] | Mesmin B, Bigay J, Polidori J, Jamecna D, Lacas-Gervais S, Antonny B (2017). Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OS- BP. EMBO J 36, 3156-3174. |
| [19] | Misteli T (2001). Protein dynamics: implications for nuclear architecture and gene expression. Science 291, 843-847. |
| [20] | Munro S (2003). Lipid rafts: elusive or illusive? Cell 115, 377-388. |
| [21] | Niko Y, Didier P, Mely Y, Konishi GI, Klymchenko AS (2016). Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes. Sci Rep 6, 18870. |
| [22] | Niko Y, Kawauchi S, Konishi GI (2013). Solvatochromic pyrene analogues of prodan exhibiting extremely high fluorescence quantum yields in apolar and polar solvents. Chem Eur J 19, 9760-9765. |
| [23] | Parasassi T, Gratton E, Yu WM, Wilson P, Levi M (1997). Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys J 72, 2413-2429. |
| [24] | Roche Y, Gerbeau-Pissot P, Buhot B, Thomas D, Bonneau L, Gresti J, Mongrand S, Perrier-Cornet JM, Simon-Plas F (2008). Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB J 22, 3980-3991. |
| [25] | Sezgin E, Sadowski T, Simons K (2014). Measuring lipid packing of model and cellular membranes with environment sensitive probes. Langmuir 30, 8160-8166. |
| [26] | Shaw JE, Epand RF, Epand RM, Li ZG, Bittman R, Yip CM (2006). Correlated fluorescence-atomic force microscopy of membrane domains: structure of fluorescence probes determines lipid localization. Biophys J 90, 2170-2178. |
| [27] | Simons K, Ikonen E (1997). Functional rafts in cell membranes. Nature 387, 569-572. |
| [28] | Simons K, Toomre D (2000). Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1, 31-39. |
| [29] | Singer SJ, Nicolson GL (1972). The fluid mosaic model of the structure of cell membranes. Science 175, 720-731. |
| [30] | Tang L, Li Y, Zhong C, Deng X, Wang XH (2021). Plant sterol clustering correlates with membrane microdomains as revealed by optical and computational microscopy. Mem- branes 11, 747. |
| [31] | Yan X, Xu M, Wang YT, Pan WH, Pan JW, Shou JX, Wang C (2022). Coupling regulation of endocytosis and exocytosis in plants. Chin Bull Bot 57, 375-387. (in Chinese) |
| 严旭, 徐梅, 王玉同, 潘伟槐, 潘建伟, 寿建昕, 王超 (2022). 植物胞吞和胞吐的耦合调控. 植物学报 57, 375-387. | |
| [32] | Zhang L, Xing JJ, Lin JX (2019). At the intersection of exocytosis and endocytosis in plants. New Phytol 224, 1479-1489. |
| [33] | Zhao XY (2014). Application of di-4-ANEPPDHQ as a Novel Fluorescent Probe for Visualization and Detection of Membrane Microdomains in Living Cells in Arabidopsis thaliana. Master’s thesis. Beijing: Beijing Forestry University. pp. 1-82. (in Chinese) |
| [34] | 赵晓玉 (2014). 新型荧光探针di-4-ANEPPDHQ在拟南芥质膜微区显微成像和定量检测中的应用. 硕士论文. 北京: 北京林业大学. pp. 1-82. |
| [35] | Zuo CS, Liu DY, Xu QJ, Shi WZ, Niu J, Chass GC (2013). Research progress on structure and function of phytosterols. J Henan Sci Technol 32, 211-213. (in Chinese) |
| 左春山, 刘大勇, 徐启杰, 时文中, 牛静, Chass GC (2013). 植物甾醇的结构与功能的研究进展. 河南科技 32, 211-213. |
/
| 〈 |
|
〉 |