Establishment of Biolistic Mediated Transformation System for Elymus sibiricus
- Pengfei Du ,
- Yu Wang ,
- Yingping Cao ,
- Song Yang ,
- Zhichao Sun ,
- Decai Mao ,
- Jiajun Yan ,
- Daxu Li ,
- Meizhen Sun ,
- Chunxiang Fu ,
- Shiqie Bai
- 1Southwest University for Nationalities, Chengdu 610041, China
2Shandong Technology Innovation Center of Synthetic Biology, Shandong Provincial Key Laboratory of Energy Genetics, Key Laboratory of Biofuels, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China
3Sichuan Grassland Science Research Institute, Chengdu 611731, China
4Engineering Research Center for Ecological Restoration of Alpine Grassland on the Qinghai-Tibet Plateau, National Forestry and Grassland Administration, Chengdu 611731, China
5Qingdao Blood Center, Qingdao 266071, China
Received date: 2020-11-02
Accepted date: 2021-01-05
Online published: 2021-01-05
Abstract
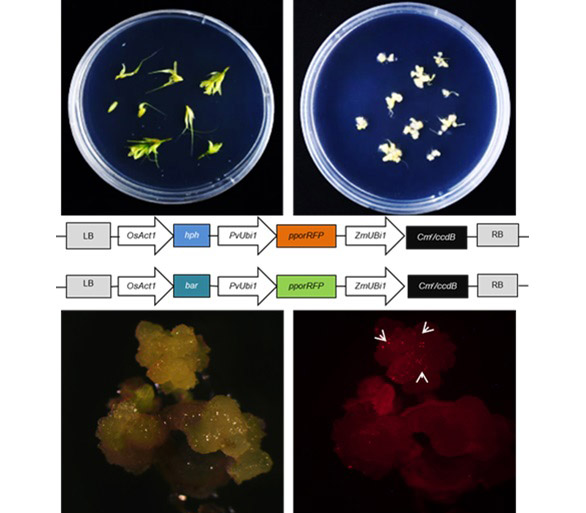
Elymus sibiricus cv. ‘Chuancao No.2’ is the main cultivated grass species for desertification control and construction of high-yield and high-quality pasture in northwest Sichuan Plateau. In this study, we tested five explants of E. sibiricus cv. ‘Chuancao No.2’ for callus induction, and found that only inflorescence calli were able to differentiate and regenerate. The calli of inflorescence with dense and hard structure cultured for 25 d and 35 d were used for Agrobacterium and biolistic mediated transformation respectively. The results showed that only biolistic-mediated transformation could produce positive transgenic calli of ‘Chuancao No.2’. In the process of biolistic-mediated transformation, the calli was pretreated in two ways: hyperosmotic culture and filter paper drying. The results revealed that the transformation efficiency of filter paper drying was higher than that of hyperosmotic treatment. For the inflorescence callus after 25 d induction, the transformation efficiency under the condition of 2 h drying of filter paper was highest which reached about 40%. In short, we applied the biolistic technology in ‘Chuancao No.2’ for the first time and successfully obtained the positive transgenic inflorescence calli. This work will lead to establishment of the robust transformation system for E. sibiricus in future.
Cite this article
Pengfei Du , Yu Wang , Yingping Cao , Song Yang , Zhichao Sun , Decai Mao , Jiajun Yan , Daxu Li , Meizhen Sun , Chunxiang Fu , Shiqie Bai . Establishment of Biolistic Mediated Transformation System for Elymus sibiricus[J]. Chinese Bulletin of Botany, 2021 , 56(1) : 62 -70 . DOI: 10.11983/CBB20174
References
| [1] | 白史且, 鄢家俊 (2020). 老芒麦种质资源研究与利用. 北京: 科学出版社. pp. 21-26. |
| [2] | 陈默君, 贾慎修 (2002). 中国饲用植物. 北京: 中国农业出版社. pp. 52-68. |
| [3] | 郭夏宇, 李合松, 彭克勤, 黄志刚, 萧浪涛 (2011). 南荻的组织培养与快速繁殖技术. 植物生理学报 47, 987-990. |
| [4] | 霍秀文, 魏建华, 张辉, 米福贵, 云锦凤 (2004). 冰草属植物组织培养再生体系的建立. 华北农学报 19, 17-20. |
| [5] | 李才旺, 柏正强, 曹毅, 汤茂林 (1999). 提高多年生人工草地建植当年产草量的研究. 四川草原 ( 1), 5-7. |
| [6] | 李达旭 (2006). 几种禾本科牧草遗传转化体系的建立和抗虫转基因研究. 博士论文. 成都: 四川大学. pp. 85-123. |
| [7] | 李达旭, 张杰, 赵建, 张艺, 李力, 刘素君, 陈飞, 杨志荣 (2006). 根癌农杆菌介导转化川草二号老芒麦胚性愈伤组织. 植物生理与分子生物学学报 32, 45-51. |
| [8] | 王关林, 方宏筠 (2002). 植物基因工程(第2版). 北京: 科学出版社. pp. 321-327. |
| [9] | 王宇 (2014). 几种牧草再生体系和遗传转化体系的优化. 硕士论文. 兰州: 兰州大学. pp. 46-51. |
| [10] | 王元富, 杨智永, 盘朝邦 (1995). 川草2号老芒麦选育报告. 四川草原 ( 1), 19-24. |
| [11] | 吴召林, 祁娟, 刘文辉, 金鑫, 杨航, 宿敬龙, 李明 (2020). 氮素形态及其配比对老芒麦生长及生理特性的影响. 草业科学 37, 942-951. |
| [12] | 鄢家俊, 白史且, 马啸, 干友民, 张建波 (2007). 老芒麦遗传多样性及育种研究进展. 植物学通报 24, 226-231. |
| [13] | 杨静 (2019). 根癌农杆菌介导单子叶植物遗传转化研究进展. 种子科技 37(18), 10-12. |
| [14] | 喻修道, 徐兆师, 陈明, 李连城, 马有志 (2010). 小麦转基因技术研究及其应用. 中国农业科学 43, 1539-1553. |
| [15] | 张童 (2009). 禾本科植物离体再生体系研究进展. 中国新技术新产品 ( 1), 169. |
| [16] | 周国栋, 李志勇, 李鸿雁, 师文贵, 李兴酉, 刘磊, 韩海波 (2011). 老芒麦种质资源的研究进展. 草业科学 28, 2026-2031. |
| [17] | 周妍彤, 张琳, 郭强, 田小霞, 孟林, 崔国文 (2018). 长穗偃麦草幼穗离体培养高频再生体系的建立. 植物生理学报 54, 1475-1480. |
| [18] | Chen KL, Gao CX (2015). Developing CRISPR technology in major crop plants. In: Zhang F, Puchta H, Thomson J, eds. Advances in New Technology for Targeted Modification of Plant Genomes. New York: Springer. pp. 145-159. |
| [19] | Cheng M, Lowe BA, Spencer TM, Ye XD, Armstrong CL (2004). Factors influencing Agrobacterium-mediated transformation of monocotyledonous species. In Vitro Cell Dev Biol Plant 40, 31-45. |
| [20] | De Cleene M, de Ley J (1976). The host range of crown gall. Bot Rev 42, 389-466. |
| [21] | Feng ZY, Zhang BT, Ding WN, Liu XD, Yang DL, Wei PL, Cao FQ, Zhu SH, Zhang F, Mao YF, Zhu JK (2013). Efficient genome editing in plants using a CRISPR/Cas system. Cell Res 23, 1229-1232. |
| [22] | Lee KW, Chinzorig O, Choi GJ, Kim KY, Ji HC, Park HS, Lee SH (2012). Callus induction and plant regeneration from mature seeds of Siberian wildrye grass (Elymus sibiricus L.). J Anim Plant Sci 22, 518-521. |
| [23] | Li DX, Zhang J, Zhao J, Zhang Y, Chen F, Zhu JQ, Liu SJ, Yang ZR (2006). Plant regeneration via somatic embryogenesis of Elymus sibiricus cv. ‘Chuancao No.2’. Plant Cell Tissue Organ Cult 84, 285-292. |
| [24] | Li JF, Norville JE, Aach J, McCormack M, Zhang DD, Bush J, Church GM, Sheen J (2013). Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31, 688-691. |
| [25] | Liang Z, Zhang K, Chen KL, Gao CX (2014). Targeted mutagenesis in Zea mays using TALENs and the CRISPR/ Cas system. J Genet Genomics 41, 63-68. |
| [26] | Mann DGJ, Lafayette PR, Abercrombie LL, King ZR, Mazarei M, Halter MC, Poovaiah CR, Baxter H, Shen H, Dixon RA, Parrott WA, Stewart CN Jr (2012). Gateway-compatible vectors for high-throughput gene functional analysis in switchgrass (Panicum virgatum L.) and other monocot species. Plant Biotechnol J 10, 226-236. |
| [27] | Mao YF, Zhang H, Xu NF, Zhang BT, Gao F, Zhu JK (2013). Application of the CRISPR-Cas system for efficient genome engineering in plants. Mol Plant 6, 2008-2011. |
| [28] | McGuire PE, Dv?rák J (1981). High salt tolerance potential in wheatgrasses. Crop Sci 21, 702-705. |
| [29] | Shan QW, Wang YP, Li J, Zhang Y, Chen KL, Liang Z, Zhang K, Liu JX, Xi JJ, Qiu JL, Gao CX (2013). Targeted genome modification of crop plants using a CRISPR- Cas system. Nat Biotechnol 31, 686-688. |
| [30] | Vasil IK (1994). Molecular improvement of cereals. Plant Mol Biol 25, 925-937. |
| [31] | Wang ZY, Ge YX (2005). Agrobacterium-mediated high efficiency transformation of tall fescue (Festuca arundinacea). J Plant Physiol 162, 103-113. |
| [32] | Wang ZY, Ge YX (2006). Recent advances in genetic transformation of forage and turf grasses. In Vitro Cell Dev Biol Plant 42, 1-18. |