玉米ZmICE2基因调控气孔发育
- 1甘肃省农业科学院作物研究所, 兰州 730070
2河南大学生命科学学院, 省部共建作物逆境适应与改良国家重点实验室, 开封 475004
收稿日期: 2022-11-14
录用日期: 2023-04-18
网络出版日期: 2023-05-23
基金资助
河南大学省部共建作物逆境适应与改良国家重点实验室开放课题(2021KF04);兰州大学细胞活动与逆境适应教育部重点实验室开放基金(lzujbky-2022-kb03);国家自然科学基金(32160490);国家自然科学基金(31860384);甘肃省重大专项计划(21ZD11NA005);甘肃省重大专项计划(21ZD10NF003)
ZmICE2 Regulates Stomatal Development in Maize
- Wenqi Zhou ,
- Yuqian Zhou ,
- Yongsheng Li ,
- Haijun He ,
- Yanzhong Yang ,
- Xiaojuan Wang ,
- Xiaorong Lian ,
- Zhongxiang Liu ,
- Zhubing Hu
- 1Crops Research Institute, Gansu Academy of Agricultural Sciences, Lanzhou 730070, China
2State Key Laboratory of Crop Stress Adaptation and Improvement, School of Life Sciences, Henan University, Kaifeng 475004, China
Received date: 2022-11-14
Accepted date: 2023-04-18
Online published: 2023-05-23
摘要
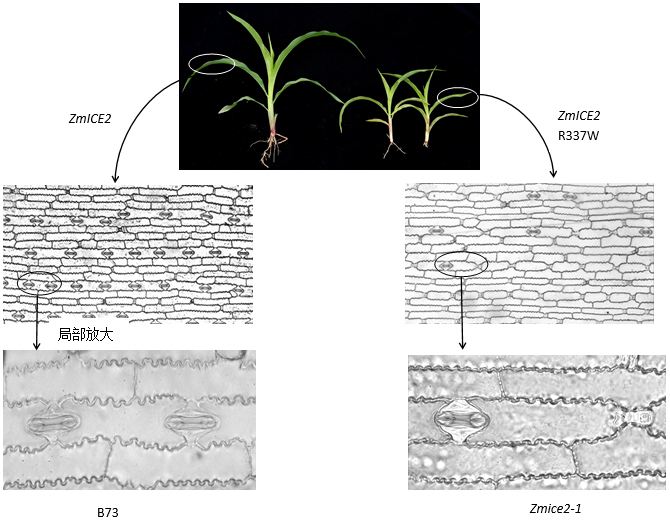
植物表皮在调节光合作用、呼吸作用、热量散失和水分利用等方面发挥重要作用。在拟南芥(Arabidopsis thaliana)等双子叶植物中, 气孔发育机理研究取得显著进展, 报道了3个非常重要的bHLH正调控转录因子(SPCH、MUTE和FAMA), 它们在气孔系细胞分裂与分化的不同阶段特异表达, 分别与转录因子SCRM/ICE1和SCRM2/ICE2形成异二聚体, 共同调控气孔细胞系在3个分裂阶段的细胞形态转换和变化, 最终发育形成气孔复合体。然而, 在单子叶植物尤其是禾本科植物玉米(Zea mays)中, 调控表皮形态建成的基因研究较少。该文利用反向遗传学手段分离到2个单基因隐性遗传突变体Zmice1-1 (inducer of cbf expression1-1)和Zmice2-1, 与对照B73相比, Zmice2-1植株矮小, 叶片黄化, 育性降低, 叶片气孔密度和气孔指数极显著降低, 打破了1个气孔间隔1个表皮长细胞的排列模式; Zmice1-1从五叶一心期开始叶片逐渐发黄, 后期全部黄化, 生长停滞, 纯合不育, 其叶片气孔密度与对照无显著差异。利用CRISPR-Cas9基因编辑技术获得不同位点的等位突变体, 表型鉴定发现Zmice2-2具有气孔异常表型, 并且与Zmice2-1的气孔表型类似, 表明ZmICE2参与调控气孔发育。B73和Zmice2-1的转录组分析表明, ZmICE2主要通过影响细胞分裂和分化来调控气孔发育, 参与玉米表皮形态建成。研究结果有助于进一步完善玉米表皮形态建成机制, 并为提高农作物的抗逆性和产量性状的遗传改良提供了有益的基因资源。
关键词: 玉米; 气孔发育; 气孔密度; 表皮形态建成; CRISPR-Cas9基因编辑
本文引用格式
周文期 , 周玉乾 , 李永生 , 何海军 , 杨彦忠 , 王晓娟 , 连晓荣 , 刘忠祥 , 胡筑兵 . 玉米ZmICE2基因调控气孔发育[J]. 植物学报, 2023 , 58(6) : 866 -881 . DOI: 10.11983/CBB22261
Abstract
Plant epidermis is crucial in regulating photosynthesis, respiration, heat dissipation, and water utilization. Significant progress has been made in the study of stomatal development in dicotyledonous plants, such as Arabidopsis thaliana. Three important bHLH transcription factors (SPCH, MUTE, and FAMA) have been reported to be specifically expressed at different stages of cell division and differentiation in the stomatal lineage. They form heterodimers with another transcription factors SCRM/ICE1 and SCRM2/ICE2 to regulate the morphological transformation and changes of stomatal lineage cells across three stages of division, finally forming the stomatal complex. However, in monocots, especially in Poaceae plants such as maize (Zea mays), studies on genes regulating epidermal morphogenesis are less reported. In this study, two single-gene recessive mutants, Zmice1-1 (inducer of cbf expression1-1) and Zmice2-1, were isolated using reverse genetics approaches. Compared to the control B73, Zmice2-1 exhibited dwarfism, leaf chlorosis, reduced fertility, significantly lower stomatal density and index, disrupted arrangement of epidermal long cells, and absence of spacing between stomata. Zmice1-1 leaves gradually turned yellow from the five-leaf stage and displayed complete chlorosis at later stages. The homozygous Zmice1-1 plants are growth-arrested and sterile, but the stomatal density showed no significant difference compared to the control. Different allels of Zmice2 were obtained using CRISPR-Cas9 genome editing technology. Phenotypic identification showed that Zmice2-2 had an abnormal stomatal phenotype similar to Zmice2-1, indicating that ZmICE2 is involved in the regulation of stomatal development. Transcriptome analysis of B73 and Zmice2-1 revealed that ZmICE2 primarily regulated stomatal development by affecting cell division and differentiation, participating in the formation of maize epidermal morphology. These results contribute to a better understanding of the mechanisms of epidermal morphogenesis in maize and provide valuable genetic resources for improving crop resilience and yield traits.

参考文献
| [1] | 陈亮, 侯岁稳 (2017). 植物气孔发育的分子遗传调控. 中国科学: 生命科学 47, 798-807. |
| [2] | 陈青云 (2017). 玉米中STOMAGEN-Like基因调控气孔发育的功能研究. 硕士论文. 南宁: 广西大学. pp. 35-60. |
| [3] | 刘延波, 项阳, 秦利军, 赵德刚 (2014). 转玉米ZmSDD1基因烟草降低气孔密度提高抗旱性. 植物生理学报 50, 1889-1898. |
| [4] | 牛艳丽, 柏胜龙, 王麒云, 刘凌云 (2017). 单细胞组学技术及其在植物保卫细胞研究中的应用. 植物学报 52, 788-796. |
| [5] | 商业绯, 李明, 丁博, 牛浩, 杨振宁, 陈小强, 曹高燚, 谢晓东 (2017). 生长素调控植物气孔发育的研究进展. 植物学报 52, 235-240. |
| [6] | 王宏亮, 郭思义, 王棚涛, 宋纯鹏 (2018). 植物气孔发育机制研究进展. 植物学报 53, 164-174. |
| [7] | 张一弓, 张怡, 张怡, 阿依白合热木·木台力甫, 张道远 (2021). 异源过表达齿肋赤藓ScABI3基因改变拟南芥气孔表型并提高抗旱性. 植物学报 56, 414-421. |
| [8] | 周文期 (2015). 调控水稻叶表皮发育的LPL2和DSP1基因克隆与功能分析. 博士论文. 兰州: 兰州大学. pp. 38-66. |
| [9] | 周文期, 寇思荣, 连晓荣, 杨彦忠, 刘忠祥, 王晓娟, 何海军, 周玉乾 (2020a). 水稻和玉米叶表皮突变体的筛选和鉴定. 植物生理学报 56, 189-199. |
| [10] | 周文期, 连晓荣, 周玉乾, 王兴荣, 杨彦忠, 刘忠祥, 王晓娟, 何海军, 寇思荣 (2020b). EMS诱变玉米自交系种质创新应用. 玉米科学 28(6), 31-38. |
| [11] | 周玉乾, 孟思远, 周文期 (2018). 植物表皮形态建成的分子调控机制. 西北农学报 27, 609-616. |
| [12] | 周文期, 强晓霞, 王森, 江静雯, 卫万荣 (2022). 水稻OsLPL2/PIR基因抗旱耐盐机制研究. 作物学报 48, 1401-1415. |
| [13] | 周玉萍, 颜嘉豪, 田长恩 (2022). 保卫细胞中ABA信号调控机制研究进展. 植物学报 57, 684-696. |
| [14] | Bergmann DC, Sack FD (2007). Stomatal development. Annu Rev Plant Biol 58, 163-181. |
| [15] | Caine RS, Yin XJ, Sloan J, Harrison EL, Mohammed U, Fulton T, Biswal AK, Dionora J, Chater CC, Coe RA, Bandyopadhyay A, Murchie EH, Swarup R, Quick WP, Gray JE (2019). Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol 221, 371-384. |
| [16] | Chater CCC, Caine RS, Fleming AJ, Gray JE (2017). Origins and evolution of stomatal development. Plant Physiol 174, 624-638. |
| [17] | Chen ZH, Chen G, Dai F, Wang YZ, Hills A, Ruan YL, Zhang GP, Franks PJ, Nevo E, Blatt MR (2017). Molecular evolution of grass stomata. Trends Plant Sci 22, 124-139. |
| [18] | Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong XH, Agarwal M, Zhu JK (2003). ICE1: a regulator of cold- induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17, 1043-1054. |
| [19] | Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS (2007). Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol 176, 275-287. |
| [20] | Deng CY, Ye HY, Fan M, Pu TL, Yan JB (2017). The rice transcription factors OsICE confer enhanced cold tolerance in transgenic Arabidopsis. Plant Signal Behav 12, e1316442. |
| [21] | Dong CH, Agarwal M, Zhang YY, Xie Q, Zhu JK (2006). The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA 103, 8281-8286. |
| [22] | Edwards D, Kerp H, Hass H (1998). Stomata in early land plants: an anatomical and ecophysiological approach. J Exp Bot 49, 255-278. |
| [23] | Feng F, Qi WW, Lv YD, Yan SM, Xu LM, Yang WY, Yuan Y, Chen YH, Zhao H, Song RT (2018). OPAQUE11 is a central hub of the regulatory network for maize endosperm development and nutrient metabolism. Plant Cell 30, 375-396. |
| [24] | Frank MJ, Smith LG (2002). A small, novel protein highly conserved in plants and animals promotes the polarized growth and division of maize leaf epidermal cells. Curr Biol 12, 849-853. |
| [25] | Gao Y, Wu MQ, Zhang MJ, Jiang W, Ren XY, Liang EX, Zhang DP, Zhang CQ, Xiao N, Li Y, Dai Y, Chen JM (2018). A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant Biotechnol J 16, 1375-1387. |
| [26] | Han SK, Qi XY, Sugihara K, Dang JH, Endo TA, Miller KL, Kim ED, Miura T, Torii KU (2018). MUTE directly orchestrates cell-state switch and the single symmetric division to create stomata. Dev Cell 45, 303-315. |
| [27] | Han SK, Torii KU (2019). Linking cell cycle to stomatal differentiation. Curr Opin Plant Biol 51, 66-73. |
| [28] | Hepworth C, Caine RS, Harrison EL, Sloan J, Gray JE (2018). Stomatal development: focusing on the grasses. Curr Opin Plant Biol 41, 1-7. |
| [29] | Jiang HF, Shi YT, Liu JY, Li Z, Fu DY, Wu SF, Li MZ, Yang ZJ, Shi YL, Lai JS, Yang XH, Gong ZZ, Hua J, Yang SH (2022). Natural polymorphism of ZmICE1 contributes to amino acid metabolism that impacts cold tolerance in maize. Nat Plants 8, 1176-1190. |
| [30] | Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu JK, Torii KU (2008). SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell 20, 1775-1785. |
| [31] | Kidokoro S, Kim JS, Ishikawa T, Suzuki T, Shinozaki K, Yamaguchi-Shinozaki K (2020). DREB1A/CBF3 is repressed by transgene-induced DNA methylation in the Arabidopsis ice1-1 mutant. Plant Cell 32, 1035-1048. |
| [32] | Lau OS, Bergmann DC (2012). Stomatal development: a plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 139, 3683-3692. |
| [33] | Li XM, Han HP, Chen M, Yang W, Liu L, Li N, Ding XH, Chu ZH (2017). Overexpression of OsDT11, which encodes a novel cysteine-rich peptide, enhances drought tolerance and increases ABA concentration in rice. Plant Mol Biol 93, 21-34. |
| [34] | Liu T, Ohashi-Ito K, Bergmann DC (2009). Orthologs of Arabidopsis thaliana stomatal bHLH genes and regulation of stomatal development in grasses. Development 136, 2265-2276. |
| [35] | Lu XD, Liu JS, Ren W, Yang Q, Chai ZG, Chen RM, Wang L, Zhao J, Lang ZH, Wang HY, Fan YL, Zhao JR, Zhang CY (2018). Gene-indexed mutations in maize. Mol Plant 11, 496-504. |
| [36] | MacAlister CA, Ohashi-Ito K, Bergmann DC (2007). Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445, 537-540. |
| [37] | Matos JL, Lau OS, Hachez C, Cruz-Ramírez A, Scheres B, Bergmann DC (2014). Irreversible fate commitment in the Arabidopsis stomatal lineage requires a FAMA and RETINOBLASTOMA-RELATED module. eLife 3, e03271. |
| [38] | McKown KH, Bergmann DC (2020). Stomatal development in the grasses: lessons from models and crops (and crop models). New Phytol 227, 1636-1648. |
| [39] | Miura K, Jin JB, Lee J, Yoo CY, Stirm V, Miura T, Ashworth EN, Bressan RA, Yun DJ, Hasegawa PM (2007). SIZ1-mediated sumoylation of ICE1 controls CBF3/ DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19, 1403-1414. |
| [40] | Nadeau JA, Sack FD (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296, 1697-1700. |
| [41] | Ohashi-Ito K, Bergmann DC (2006). Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18, 2493-2505. |
| [42] | Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU (2007). Termination of asymmetric cell division and differentiation of stomata. Nature 445, 501-505. |
| [43] | Pillitteri LJ, Torii KU (2012). Mechanisms of stomatal development. Annu Rev Plant Biol 63, 591-614. |
| [44] | Putarjunan A, Ruble J, Srivastava A, Zhao CZ, Rychel AL, Hofstetter AK, Tang XB, Zhu JK, Tama F, Zheng N, Torii KU (2019). Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat Plants 5, 742-754. |
| [45] | Qu X, Peterson KM, Torii KU (2017). Stomatal development in time: the past and the future. Curr Opin Genes Dev 45, 1-9. |
| [46] | Raissig MT, Abrash E, Bettadapur A, Vogel JP, Bergmann DC (2016). Grasses use an alternatively wired bHLH transcription factor network to establish stomatal identity. Proc Natl Acad Sci USA 113, 8326-8331. |
| [47] | Raissig MT, Matos JL, Ximena Anleu Gil M, Kornfeld A, Bettadapur A, Abrash E, Allison HR, Badgley G, Vogel JP, Berry JA, Bergmann DC (2017). Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 355, 1215-1218. |
| [48] | Rudall PJ, Hilton J, Bateman RM (2013). Several developmental and morphogenetic factors govern the evolution of stomatal patterning in land plants. New Phytol 200, 598-614. |
| [49] | Serna L (2011). Stomatal development in Arabidopsis and grasses: differences and commonalities. Int J Dev Biol 55, 5-10. |
| [50] | Serna L (2020). The role of grass MUTE orthologues during stomatal development. Front Plant Sci 11, 55. |
| [51] | Wang HL, Guo SY, Qiao X, Guo JF, Li ZL, Zhou YS, Bai SL, Gao ZY, Wang DJ, Wang PC, Galbraith DW, Song CP (2019). BZU2/ZmMUTE controls symmetrical division of guard mother cell and specifies neighbor cell fate in maize. PLoS Genet 15, e1008377. |
| [52] | Wei DH, Liu MJ, Chen H, Zheng Y, Liu YX, Wang X, Yang SH, Zhou MQ, Lin J (2018). INDUCER OF CBF EXPRESSION 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genet 14, e1007695. |
| [53] | Wu ZL, Chen L, Yu Q, Zhou WQ, Gou XP, Li J, Hou SW (2019). Multiple transcriptional factors control stomata development in rice. New Phytol 223, 220-232. |
| [54] | Ye KY, Li H, Ding YL, Shi YT, Song CP, Gong ZZ, Yang SH (2019). BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 31, 2682-2696. |
| [55] | Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV (2010). The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22, 4128-4141. |
| [56] | Zhou WQ, Wang YC, Wu ZL, Luo L, Liu P, Yan LF, Hou SW (2016). Homologs of SCAR/WAVE complex components are required for epidermal cell morphogenesis in rice. J Exp Bot 67, 4311-4323. |
| [57] | Zoulias N, Harrison EL, Casson SA, Gray JE (2018). Molecular control of stomatal development. Biochem J 475, 441-454. |