蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响
- 南京农业大学食品科学技术学院, 南京 210095
收稿日期: 2020-09-29
录用日期: 2020-12-25
网络出版日期: 2020-12-29
基金资助
中央高校基本业务费(KYZ201824);国家自然科学基金No(31871754);国家自然科学基金No(31871793)
Changes of Protein N-glycosylation in the Growth of Arabidopsis thaliana and Effects of Enzymatic Deglycosylation on Root Development
- College of Food Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
† These authors contributed equally to this paper
Received date: 2020-09-29
Accepted date: 2020-12-25
Online published: 2020-12-29
摘要
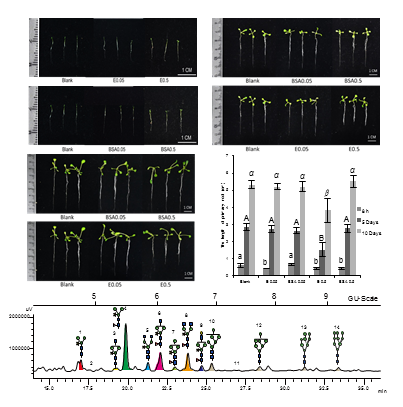
蛋白质N-糖基化修饰在植物生长发育中发挥重要作用。为探究蛋白质N-糖基化在拟南芥(Arabidopsis thaliana)整个生长周期中的变化规律以及去N-糖基化对拟南芥生根发育的影响, 通过N-糖链酶解和HPLC与MALDI-TOF-MS分析解析了不同生长时期的拟南芥Col-0植株的N-糖链组成(结构和含量)变化。以BSA溶液为阴性对照, 无菌去离子水为空白对照, 用N-糖酰胺酶(PNGase Rz)溶液处理拟南芥幼苗8小时; 然后继续在MS培养基中培养5天、10天, 测量主根长度并检测N-糖链组成的变化。结果显示, 从拟南芥中解析出12种N-糖链结构, 其中包括4个高甘露糖型和8个复杂型。在拟南芥整个生长周期中, 复杂型N-糖链含量始终高于高甘露糖型, 其中含木糖和岩藻糖的复杂型结构是N-糖链的主要组成, 而Man3XylFucGlcNAc2含量最高。高甘露糖型N-糖链含量由幼苗期的13.87%缓慢上升至抽薹期的19.02%, 盛花期回落至17.98%, 而在长角果成熟期快速下降至最低点2.36%, 衰老期再度小幅回升至5.23%。用高浓度糖酰胺酶液PNGase Rz处理后, 可观察到幼苗主根生长受到显著抑制, 且培养10天后仍然无法恢复正常; 而低浓度酶液处理组与阴性对照组差异不显著, 根长和生长状态基本正常。糖链分析结果显示, 与对照组相比, 高、低浓度酶液处理组的N-糖链组成均发生显著变化, 主要表现为高甘露糖型含量显著低于空白对照组, 同时随生长时间的延长该差异逐渐减小, 最终消失。研究表明, 拟南芥N-糖基化组成随着生长发育发生周期性变化, 且去糖基化酶处理能够瞬时影响拟南芥蛋白质N-糖基化修饰, 进而抑制根的发育。
本文引用格式
王婷 , 羊欢欢 , 赵弘巍 , JosefVoglmeir , 刘丽 . 蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响[J]. 植物学报, 2021 , 56(3) : 262 -274 . DOI: 10.11983/CBB20163
Abstract
N-glycosylation of proteins plays an important role in plant growth and development. This study explored the changes in protein N-glycosylation in Arabidopsis thaliana at different growth stages and its role in the root development. N-glycans of Arabidopsis Col-0 plants at different growth stages were enzymatically released with N-glycanase and analyzed by HPLC and MALDI-TOF-MS. In addition, Arabidopsis seedlings were treated with N-glycanase, PNGase Rz, for 8 hours before further cultivation in MS medium for five and ten days. The group treated with BSA solution was used as the negative control and the group treated with sterile deionized water was used as blank control. The changes in the primary root length and N-glycosylation composition of the seedlings were measured after treatment. A total number of 12 N-glycan structures were deduced from Arabidopsis, including 4 high-mannose types and 8 complex types. Throughout the entire period, the content of complex type N-glycan was always higher than that of high-mannose; the complex structures modified with xylose and fucose were the dominant component, among which Man3XylFucGlcNAc2 is the highest. The changes of high-mannose N-glycan were as follows: the content steadily increased from 13.87% (seedling stage) to 19.02% (bolting stage), slightly decreased to 17.98% (flowering stage), and dramatically dropped to 2.36% (longhorn ripening stage), then returned 5.23% (aging stage). After treatment with PNGase Rz at high concentration, a significant inhibition of the primary root’s growth was observed, which could not be recovered after cultivation in MS medium for ten days. However, no statistical differences of root length and growth state were found in the treatment group of low concentration (0.05 mg·mL -1), compared with the negative group. N-glycan analysis of seedlings revealed that compared with the control, both treatment groups showed significant changes in the composition of N-glycans. Especially, the total content of high-mannose N-glycans was dramatically lower than that of the blank control group. Meanwhile, these glycoform differences shrank with prolonged time of cultivation and finally disappeared. In conclusion, Arabidopsis thaliana has unique pattern of N-glycosylation during the whole growing period; and glycanase treatment could intermediately alter the N-glycosylation pattern and subsequently inhibit the development of root.

Key words: Arabidopsis thaliana; N-glycan; root development; deglycosylation
参考文献
| 1 | 王梦, 危双, 王婷, Voglmeir J, 刘丽 (2017). Solitalea canadensis源β-N-乙酰氨基己糖苷酶的基因克隆、异源表达和酶学特性. 微生物学报 57, 1270-1282. |
| 2 | Altmann F (2007). The role of protein glycosylation in allergy. Int Arch Allergy Immunol 142, 99-115. |
| 3 | Bao LG, Ma SW, van Huystee RB (2001). The effects of the site-directed removal of N-glycosylation from cationic peanut peroxidase on its function. Arch Biochem Biophys 386, 17-24. |
| 4 | Dam S, Thaysen-Andersen M, Stenkj?r E, Lorentzen A, Roepstorff P, Packer NH, Stougaard J (2013). Combined N-glycome and N-glycoproteome analysis of the Lotus japonicus seed globulin fraction shows conservation of protein structure and glycosylation in legumes. J Proteome Res 12, 3383-3392. |
| 5 | Du YM, Xia T, Gu XQ, Wang T, Ma HY, Voglmeir J, Liu L (2015). Rapid sample preparation methodology for plant N-glycan analysis using acid-stable PNGase H+. J Agric Food Chem 63, 10550-10555. |
| 6 | Ghazarian H, Idoni B, Oppenheimer SB (2011). A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem 113, 236-247. |
| 7 | Horiuchi R, Hirotsu N, Miyanishi N (2015). Comparative analysis of N-glycans in the ungerminated and germinated stages of Oryza sativa. Carbohydr Res 418, 1-8. |
| 8 | Ihara Y, Ikezaki M, Takatani M, Ito Y (2020). Calnexin/calreticulin and assays related to N-glycoprotein folding in vitro. In: Hirabayashi J, ed. Lectin Purification and Analysis: Methods and Protocols. Humana: Springer. pp. 295-308. |
| 9 | Kajiura H, Koiwa H, Nakazawa Y, Okazawa A, Kobayashi A, Seki T, Fujiyama K (2010). Two Arabidopsis thaliana Golgi, α-mannosidase I enzymes are responsible for plant N-glycan maturation. Glycobiology 20, 235-247. |
| 10 | Kang JS, Frank J, Kang CH, Kajiura H, Vikram M, Ueda A, Kim S, Bahk JD, Triplett B, Fujiyama K, Lee SY, von Schaewen A, Koiwa H (2008). Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc Natl Acad Sci USA 105, 5933-5938. |
| 11 | Kato S, Hayashi M, Kitagawa M, Kajiura H, Maeda M, Kimura Y, Igarashi K, Kasahara M, Ishimizu T (2018). Degradation pathway of plant complex-type N-glycans: identification and characterization of a key α1,3-fucosidase from glycoside hydrolase family 29. Biochem J 475, 305-317. |
| 12 | Kimura Y, Matsuo S (2000). Changes in N-linked oligosaccharides during seed development of Ginkgo biloba. Biosci Biotechnol Biochem 64, 562-568. |
| 13 | Kimura Y, Takeoka Y, Inoue M, Maeda M, Fujiyama K (2011). Double-knockout of putative endo-β-N-acetylglucosaminidase (ENGase) genes in Arabidopsis thaliana: loss of ENGase activity induced accumulation of high- mannose type free N-glycans bearing N,N'-acetylchitobiosyl unit. Biosci Biotechnol Biochem 75, 1019-1021. |
| 14 | Koiwa H, Li F, McCully MG, Mendoza I, Koizumi N, Manabe Y, Nakagawa Y, Zhu JH, Rus A, Pardo JM, Bressan RA, Hasegawa PM (2003). The STT3a subunit isoform of the Arabidopsis oligosaccharyltransferase controls adaptive responses to salt/osmotic stress. Plant Cell 15, 2273-2284. |
| 15 | Liebminger E, Hüttner S, Vavra U, Fischl R, Schoberer J, Grass J, Blaukopf C, Seifert GJ, Altmann F, Mach L, Strasser R (2009). Class I α-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell 21, 3850-3867. |
| 16 | Liebminger E, Veit C, Pabst M, Batoux M, Zipfel C, Altmann F, Mach L, Strasser R (2011). β-N-acetylhexosaminidases HEXO1 and HEXO3 are responsible for the formation of paucimannosidic N-glycans in Arabidopsis thaliana. J Biol Chem 286, 10793-10802. |
| 17 | Lindner H, Kessler SA, Müller LM, Shimosato-Asano H, Boisson-Dernier A, Grossniklaus U (2015). TURAN and EVAN mediate pollen tube reception in Arabidopsis Synergids through protein glycosylation. PLoS Biol 13, e1002139. |
| 18 | Maeda M, Kimura Y (2014). Structural features of free N-glycans occurring in plants and functional features of de-N-glycosylation enzymes, ENGase, and PNGase: the presence of unusual plant complex type N-glycans. Front Plant Sci 5, 429. |
| 19 | Misaki R, Kimura Y, Fujiyama K, Seki T (2001). Glycoproteins secreted from suspension -cultured tobacco BY2 cells have distinct glycan structures from intracellular glycoproteins. Biosci Biotechnol Biochem 65, 2482-2488. |
| 20 | Nagashima Y, von Schaewen A, Koiwa H (2018). Function of N-glycosylation in plants. Plant Sci 274, 70-79. |
| 21 | Nakamura K, Inoue M, Yoshiie T, Hosoi K, Kimura Y (2008). Changes in structural features of free N-glycan and endoglycosidase activity during tomato fruit ripening. Biosci Biotechnol Biochem 72, 2936-2945. |
| 22 | Rips S, Bentley N, Jeong IS, Welch JL, von Schaewen A, Koiwa H (2014). Multiple N-glycans cooperate in the subcellular targeting and functioning of Arabidopsis KORRIGAN1. Plant Cell 26, 3792-3808. |
| 23 | Saijo Y (2010). ER quality control of immune receptors and regulators in plants. Cell Microbiol 12, 716-724. |
| 24 | Shen JB, Ding Y, Gao CJ, Rojo E, Jiang LW (2014). N-linked glycosylation of AtVSR1 is important for vacuolar protein sorting in Arabidopsis. Plant J 80, 977-992. |
| 25 | Strasser R, Schoberer J, Jin CS, Gl?ssl J, Mach L, Steinkellner H (2006). Molecular cloning and characterization of Arabidopsis thaliana Golgi α-mannosidase II, a key enzyme in the formation of complex N-glycans in plants. Plant J 45, 789-803. |
| 26 | Vi?tor R, Loutelier-Bourhis C, Fitchette AC, Margerie P, Gonneau M, Faye L, Lerouge P (2003). Protein N-glycosylation is similar in the moss Physcomitrella patens and in higher plants. Planta 218, 269-275. |
| 27 | Wang T, Hu XC, Cai ZP, Voglmeir J, Liu L (2017a). Qualitative and quantitative analysis of carbohydrate modification on glycoproteins from seeds of Ginkgo biloba. J Agric Food Chem 65, 7669-7679. |
| 28 | Wang T, Voglmeir J (2014). PNGases as valuable tools in glycoprotein analysis. Protein Pept Lett 21, 976-985. |
| 29 | Wang WL, Wang W, Du YM, Wu H, Yu XB, Ye KP, Li CB, Jung YS, Qian YJ, Voglmeir J, Liu L (2017b). Comparison of anti-pathogenic activities of the human and bovine milk N-glycome: fucosylation is a key factor. Food Chem 235, 167-174. |
| 30 | Wang ZY, Gehring C, Zhu JH, Li FM, Zhu JK, Xiong LM (2015). The Arabidopsis Vacuolar Sorting Receptor 1 is required for osmotic stress-induced abscisic acid biosynthesis. Plant Physiol 167, 137-152. |
| 31 | Wei S, Henquet MGL, Mentink RA, van Dijk AJ, Cordewener JHG, Bosch D, America AHP, van der Krol AR (2011). N-glycoproteomics in plants: perspectives and challenges. J Proteomics 74, 1463-1474. |
| 32 | Wilson IBH, Zeleny R, Kolarich D, Staudacher E, Stroop CJM, Kamerling JP, Altmann F (2001). Analysis of Asn -linked glycans from vegetable foodstuffs: widespread occurrence of Lewis a, core α1,3-linked fucose and xylose substitutions. Glycobiology 11, 261-274. |
| 33 | Xu SL, Medzihradszky KF, Wang ZY, Burlingame AL, Chalkley RJ (2016). N-glycopeptide profiling in Arabidopsis inflorescence. Mol Cell Proteomics 15, 2048-2054. |
| 34 | Zeng W, Ford KL, Bacic A, Heazlewood JL (2018). N- linked glycan micro-heterogeneity in glycoproteins of Arabidopsis. Mol Cell Proteomics 17, 413-421. |