地衣芽孢杆菌耐高温α-淀粉酶基因的克隆、烟草瞬时表达及转化拟南芥的研究
- 李潇 ,
- 孙海彦 ,
- 王霈虹 ,
- 彭明 ,
- 阮孟斌
- 1海南大学, 海口 570228
2中国热带农业科学院热带生物技术研究所, 海口 571101
3北京市科学技术情报研究所, 北京 100048
? 共同第一作者
收稿日期: 2014-11-15
录用日期: 2015-01-27
网络出版日期: 2015-04-08
基金资助
国家高技术研究发展计划重点项目(No.2012AA101204)、国家自然科学基金(No.31000029)、海南省自然科学基金(No.ZDFD 20120765)、海南省引进集成应用专项(No.YJJC2011004)、海南省研究生创新科研课题(No.Hyb2011-4)和海南省重大科技项目(No.ZDZX2013023-1)
Studies of the Transient Expression and Transformation of Cloned Thermostable α-Amylase Genes from Bacillus licheniformis in Tobacco and Arabidopsis
- Xiao Li ,
- Haiyan Sun ,
- Peihong Wang ,
- Ming Peng ,
- Mengbin Ruan
- 1Hainan University, Haikou 570228, China
2Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, China
3Beijing Institute of Science and Technology Information, Beijing 100048, China
? These authors contributed equally to this paper
Received date: 2014-11-15
Accepted date: 2015-01-27
Online published: 2015-04-08
摘要
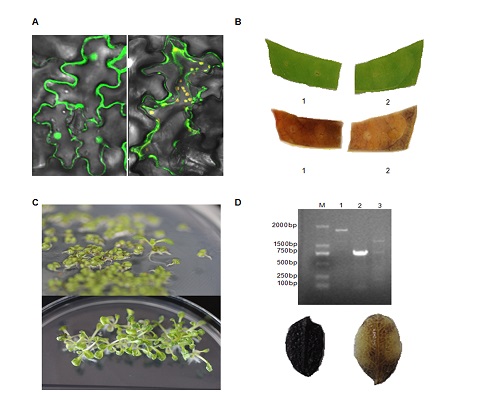
从地衣芽孢杆菌(Bacillus licheniformis)中克隆到耐高温α-淀粉酶基因全长, 构建了原核表达载体, 转入大肠杆菌(Escherichia coli)中, 使用IPTG于28°C诱导6小时后, 通过SDS-PAGE检测到目的蛋白, 分子量约为55 kDa, 并通过酶活力检测实验证明该蛋白具有耐高温α-淀粉酶活性。同时构建了该基因融合GFP的植物表达载体, 通过农杆菌(Agro- bacterium tumefaciens)介导瞬时转化烟草(Nicotiana tabacum)下表皮细胞并在荧光显微镜下观察, 发现在烟草下表皮细胞的细胞质和液泡中均有绿色荧光。使用I2-KI溶液对乙醇脱色后的烟草叶片进行染色, 显色反应表明在烟草中表达的耐高温α-淀粉酶具有酶活性。最后, 采用农杆菌介导的花蕾浸泡法将重组载体转化到拟南芥(Arabidopsis thaliana)中, 筛选到稳定遗传的耐高温α-淀粉酶基因的拟南芥纯合子。研究结果为后期开展表达耐高温α-淀粉酶的转基因植物的相关研究奠定了实验基础。
本文引用格式
李潇 , 孙海彦 , 王霈虹 , 彭明 , 阮孟斌 . 地衣芽孢杆菌耐高温α-淀粉酶基因的克隆、烟草瞬时表达及转化拟南芥的研究[J]. 植物学报, 2015 , 50(3) : 354 -362 . DOI: 10.3724/SP.J.1259.2015.00354
Abstract
The full-length thermostable α-amylase gene from a Bacillus licheniformis strain was cloned in a prokaryotic expression vector and a plant expression vector with GFP as reporter gene. Thermostable α-amylase was overexpressed in Escherichia coli by IPTG induction for 6 h at 28°C, protein bands at 55 kDa were detected with SDS-PAGE, and activity of the enzyme was confirmed by amylolysis experiments. The plant expression vector was transformed into tobacco via Agrobacterium tumefaciens mediated transformation. GFP expression was observed both in cytoplasm and vacuoles of the lower epidermis of tobacco by fluorescence microscopy. Staining of the tobacco leaf by I2-KI revealed that the thermostable α-amylase expressed in tobacco leaf had enzyme activity. Finally, the gene was introduced into Arabidopsis by the floral dip method and homozygous transgenic plants expressing α-amylase gene were identified. The results of this research provide further information for overexpression of thermostable α-amylase in transgenic plants.

参考文献
| 1 | 蔡恒, 陈忠军, 万红贵, 王涛, 杜连祥 (2008). 地衣芽孢杆菌α-淀粉酶信号肽的序列分析及其在大肠杆菌中的分泌特性. 华北农学报 23, 106-109. |
| 2 | 杜承, 孟宪梅, 卢士英, 潘风光, 柳增善 (2010). 地衣芽孢杆菌耐高温α-淀粉酶在毕赤酵母中的表达及其活性分析. 食品科技 35, 44-48. |
| 3 | 林必博 (2011). 耐高温α-淀粉酶的研究进展. 河北农业科学 15, 77-80. |
| 4 | 林剑, 郑舒文, 郭尽力, 鞠宝, 金海珠 (2002). 溶氧等参数对耐高温α-淀粉酶发酵的影响. 食品科学 23, 54-57. |
| 5 | 林剑, 郑舒文, 孙利芹 (2003). 温度和pH值对耐高温α-淀粉酶活力的影响. 中国食品添加剂(5), 65-67, 53. |
| 6 | 潘风光, 宋德群, 任洪林, 艾永兴, 柳增善 (2004). 地衣芽孢杆菌α-耐高温淀粉酶基因的克隆及在大肠杆菌中的表达. 沈阳农业大学学报 35, 42-44. |
| 7 | 秦亚楠, 孟宪梅, 潘风光, 孙肖明, 李凤祥, 石彦忠 (2011). 重组毕赤酵母生产耐高温α-淀粉酶发酵条件的优化. 食品科学 32, 266-269. |
| 8 | 王春铭, 雷恒毅, 王国惠, 陈桂珠, 李丕学, 黄洁妍 (2007). 耐高温α-淀粉酶菌的产酶条件、酶性质与环境应用研究. 环境科学学报 27, 600-607. |
| 9 | 王慧超, 陈今朝, 韩宗先 (2010). α-淀粉酶的研究与应用. 重庆工商大学学报(自然科学版) 27, 368-372. |
| 10 | 文学, 张宝红 (2000). 转基因抗虫棉研究现状与展望. 农业生物技术学报 8, 194-199. |
| 11 | 姚斌, 范云六 (2000). 植酸酶的分子生物学与基因工程. 生物工程学报 16, 1-5. |
| 12 | 赵淑娟, 黄祥辉, 王水平 (2000). 耐高温α-淀粉酶基因在马铃薯中的表达. 实验生物学报 33, 157-162. |
| 13 | 诸葛健, 唐是雯 (1994). 工业微生物实验技术手册. 北京: 中国轻工业出版社. |
| 14 | Clough SJ, Bent AF (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana.Plant J 16, 735-743. |
| 15 | Dai JL, Dong HZ (2011). Stem girdling influences concentrations of endogenous cytokinins and abscisic acid in relation to leaf senescence in cotton.Acta Physiol Plant 33, 1697-1705. |
| 16 | Lin KH, Fu HY, Chan CH, Lo HF, Shih MC, Chang YM, Chen LFO (2008). Generation and analyses of the trans- genic potatoes expressing heterologous thermostable β- amylase.Plant Sci 174, 649-657. |
| 17 | Liu YQ, Chen HK, Li RX (2010). Studies on the isolation and screening of α-amylase producing strain and their enzymatic properties.Agric Sci Technol 11, 56-58, 90. |
| 18 | Pen J, Molendijk L, Quax WJ, Sijmons PC, van Ooyen AJJ, van den Elzen PJM, Rietveld K, Hoekema A (1992). Production of active Bacillus licheniformis alpha-amylase in tobacco and its application in starch liquefaction.Nat Biotechnol 10, 292-296. |
| 19 | Rivera MH, López-Munguía A, Soberón X, Saab-Rincón G (2003). α-amylase from Bacillus licheniformis mutants near to the catalytic site: effects on hydrolytic and transglycosylation activity. Protein Eng 16, 505-514. |
| 20 | Rubio S, Donoso A, Pérez FJ (2014). The dormancy- breaking stimuli “chilling, hypoxia and cyanamide exposure” up-regulate the expression of α-amylase genes in grapevine buds.J Plant Physiol 171, 373-381. |
| 21 | Xu XL, Fang J, Wang W, Guo JL, Chen PN, Cheng JA, Shen ZC (2008). Expression of a bacterial α-amylase gene in transgenic rice seeds.Transgenic Res 17, 645-650. |